Effect of modified live or inactivated feline herpesvirus-1 parenteral vaccines on clinical and laboratory findings following viral challenge
- PMID: 27432436
- PMCID: PMC11104124
- DOI: 10.1177/1098612X16659333
Effect of modified live or inactivated feline herpesvirus-1 parenteral vaccines on clinical and laboratory findings following viral challenge
Abstract
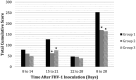
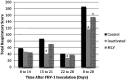
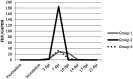
Objectives The objective was to investigate the effect of one dose of an inactivated feline herpesvirus-1 (FHV-1), feline calicivirus (FCV) and panleukopenia virus (FPV) vaccine (FVRCP) or one dose of a modified live (ML) FVRCP vaccine on clinical signs and shedding of FHV-1 in specific pathogen-free kittens after challenge with FHV-1 7 days after vaccination. Methods Twenty-four FHV-1 seronegative 5-month-old kittens were randomized into three groups of eight kittens. Group 1 kittens were maintained as unvaccinated controls, group 2 kittens were administered one dose of the inactivated FVRCP vaccine subcutaneously (SC) and group 3 kittens were administered one dose of the ML FVRCP vaccine SC. All 24 cats were administered FHV-1 by nasal and oropharyngeal inoculation 7 days later and were observed daily for clinical signs of illness for 21 days. Results In the 21 days after FHV-1 challenge, both groups of vaccinated cats were less likely to be clinically ill (indicated by lower cumulative clinical scores) than control cats ( P <0.001). There was no statistical difference in total clinical score between the two vaccinated groups ( P = 0.97). Although the total clinical score was similar between both vaccines, signs of respiratory disease were significantly fewer in the kittens vaccinated with the inactivated FVRCP vaccine compared with the ML FVRCP vaccine ( P = 0.005) during the period after inoculation when the majority of clinical disease was observed. Conclusions and relevance Parenteral administration of either the inactivated FVRCP vaccine or the ML FVRCP vaccine can decrease clinical signs of illness due to FHV-1 on a day 7 challenge when compared with controls. Use of either vaccine product is indicated in cats at risk of acute exposure to FHV-1.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Veir J, Ruch-Gallie R, Spindel ME, et al.. Prevalence of FHV-1, Mycoplasma spp., and aerobic bacteria in shelter kittens with acute upper respiratory tract disease. J Vet Intern Med 2004; 18: 375–460.
-
- Fontenelle JP, Powell CC, Veir JK, et al.. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesviurs-1 infection in cats. Am J Vet Res 2008; 69: 289–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

