Cognitive-Behavioural Therapy for Inflammatory Bowel Disease: 24-Month Data from a Randomised Controlled Trial
- PMID: 27432441
- PMCID: PMC5288424
- DOI: 10.1007/s12529-016-9580-9
Cognitive-Behavioural Therapy for Inflammatory Bowel Disease: 24-Month Data from a Randomised Controlled Trial
Abstract
Purpose: There is ongoing controversy on the effectiveness of psychotherapy in inflammatory bowel disease (IBD). In the few small studies, cognitive-behavioural therapy (CBT) has been shown to alleviate symptoms of anxiety or depression. However, there is little research on the impact of CBT on physical outcomes in IBD and no studies on long-term effectiveness of CBT.
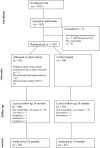
Methods: The present two-arm pragmatic randomised controlled trial aimed to establish the impact of CBT on disease course after 24 months of observation. The study compared standard care plus CBT (+CBT) with standard care alone (SC). CBT was delivered over 10 weeks, face-to-face (F2F) or online (cCBT). The data were analysed using linear mixed-effects models.
Results: CBT did not significantly influence disease activity as measured by disease activity indices at 24 months (Crohn's Disease Activity Index (CDAI), p = 0.92; Simple Clinical Colitis Activity Index (SCCAI), p = 0.88) or blood parameters (C-reactive protein (CRP), p < 0.62; haemoglobin (Hb), p = 0.77; platelet, p = 0.64; white cell count (WCC), p = 0.59) nor did CBT significantly affect mental health, coping or quality of life (all p > 0.05).
Conclusions: Therefore, we conclude that CBT does not influence the course of IBD over 24 months. Given the high rate of attrition, particularly in the CBT group, future trials should consider a personalised approach to psychotherapy, perhaps combining online and one-to-one therapist time.
Keywords: Cognitive-behavioural therapy; Disease course; Inflammatory bowel disease.
Conflict of interest statement
Compliance with Ethical Standards All authors declare that they have no competing interests to report. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Royal Adelaide Hospital and the University of South Australia Research Ethics Committees in August 2009. Informed consent was obtained from all individual participants included in the study. The privacy rights of participants were observed.
Figures
Similar articles
-
Cognitive-behavioural therapy has no effect on disease activity but improves quality of life in subgroups of patients with inflammatory bowel disease: a pilot randomised controlled trial.BMC Gastroenterol. 2015 May 2;15:54. doi: 10.1186/s12876-015-0278-2. BMC Gastroenterol. 2015. PMID: 25934170 Free PMC article. Clinical Trial.
-
Effect of Cognitive Behavioral Therapy on Clinical Disease Course in Adolescents and Young Adults With Inflammatory Bowel Disease and Subclinical Anxiety and/or Depression: Results of a Randomized Trial.Inflamm Bowel Dis. 2019 Nov 14;25(12):1945-1956. doi: 10.1093/ibd/izz073. Inflamm Bowel Dis. 2019. PMID: 31050763 Free PMC article. Clinical Trial.
-
Effectiveness of cognitive-behavioral therapy on quality of life, anxiety, and depressive symptoms among patients with inflammatory bowel disease: A multicenter randomized controlled trial.J Consult Clin Psychol. 2017 Sep;85(9):918-925. doi: 10.1037/ccp0000227. J Consult Clin Psychol. 2017. PMID: 28857595 Clinical Trial.
-
Psychotherapy for inflammatory bowel disease: a review and update.J Crohns Colitis. 2013 Dec;7(12):935-49. doi: 10.1016/j.crohns.2013.02.004. Epub 2013 Mar 5. J Crohns Colitis. 2013. PMID: 23466412 Review.
-
Systematic review with meta-analysis: online psychological interventions for mental and physical health outcomes in gastrointestinal disorders including irritable bowel syndrome and inflammatory bowel disease.Aliment Pharmacol Ther. 2018 Aug;48(3):244-259. doi: 10.1111/apt.14840. Epub 2018 Jun 14. Aliment Pharmacol Ther. 2018. PMID: 29901820
Cited by
-
Acceptance and commitment therapy for the treatment of irritable bowel syndrome and inflammatory bowel disease: a narrative review.Transl Gastroenterol Hepatol. 2024 Jun 24;9:43. doi: 10.21037/tgh-24-10. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39091663 Free PMC article. Review.
-
Patients' Experiences and Challenges in Living with Inflammatory Bowel Disease: A Qualitative Approach.Clin Exp Gastroenterol. 2021 Apr 28;14:123-131. doi: 10.2147/CEG.S303688. eCollection 2021. Clin Exp Gastroenterol. 2021. PMID: 33953591 Free PMC article.
-
The physiological and psychological effects of cognitive behavior therapy on patients with inflammatory bowel disease before COVID-19: a systematic review.BMC Gastroenterol. 2021 Dec 15;21(1):469. doi: 10.1186/s12876-021-02003-0. BMC Gastroenterol. 2021. PMID: 34911469 Free PMC article.
-
Guidelines for the management of patients with Crohn's disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology.Prz Gastroenterol. 2021;16(4):257-296. doi: 10.5114/pg.2021.110914. Epub 2021 Nov 19. Prz Gastroenterol. 2021. PMID: 34976235 Free PMC article.
-
Psychosocial Interventions and Immune System Function: A Systematic Review and Meta-analysis of Randomized Clinical Trials.JAMA Psychiatry. 2020 Oct 1;77(10):1031-1043. doi: 10.1001/jamapsychiatry.2020.0431. JAMA Psychiatry. 2020. PMID: 32492090 Free PMC article.
References
-
- CCFA. About the Epidemiology of IBD. Crohn’s and Colitis Foundation of America. 2012. http://www.ccfa.org/resources/epidemiology.html. 2015.
-
- CCA. About Crohn’s and Colitis. Crohn’s & Colitis Australia. 2015. https://www.crohnsandcolitis.com.au/about-crohns-colitis/. 2015.
-
- Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 8. Philadelphia: Saunders; 2006.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous