Molecular-targeted first-line therapy for advanced gastric cancer
- PMID: 27432490
- PMCID: PMC6457914
- DOI: 10.1002/14651858.CD011461.pub2
Molecular-targeted first-line therapy for advanced gastric cancer
Abstract
Background: Gastric cancer is the fifth most common cancer and third leading cause of cancer-related deaths worldwide. Complete resection of the whole tumor remains the only approach to treat this malignant disease. Since gastric cancer is usually asymptomatic in its early stages, many people are diagnosed at an advanced stage when the tumor is inoperable. In addition, because other conventional treatments (radiotherapy and chemotherapy) have only modest efficacy for those with advanced/metastatic gastric cancer, the prognosis in such cases is poor. Recently, trials have provided some promising results regarding molecular-targeted therapy, raising the possibility that the development of these agents could be a fruitful approach. However, the benefit of molecular-targeted therapy for advanced gastric cancer remains inconclusive.
Objectives: To evaluate the efficacy and safety of molecular-targeted therapy , either alone or in combination with chemotherapy, in people with advanced gastric cancer.
Search methods: We searched the following databases (from inception to December 2015): the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and CINAHL. In addition, we searched the reference lists of included trials and contacted experts in the field.
Selection criteria: We searched for randomized controlled trials (RCTs) in adults (aged 18 years or older) with histologically-confirmed advanced adenocarcinoma of the stomach/gastro-esophageal junction. Trials of participants with esophageal adenocarcinoma were also considered to be eligible. The eligible trials should aim to evaluate the effects of molecular-targeted agents on participants' prognosis.
Data collection and analysis: Two review authors independently performed selection of eligible trials, assessment of trial quality, and data extraction. We used methods of survival analysis and expressed the intervention effect as a hazard ratio (HR) when pooling time-to-event data, and calculated the odds ratio (OR) for dichotomous data and mean differences (MDs) for continuous data, with 95% confidence intervals (CI).
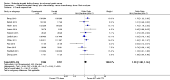
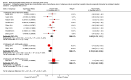
Main results: We included 11 studies randomizing 4014 participants to molecular-targeted therapy plus conventional chemotherapy or chemotherapy alone. Five were at low risk of bias, and we considered the risk of bias in the other six studies to be high, mainly due to their open-label design. All identified studies reported data regarding survival. We found low-quality evidence that molecular-targeted may have a small effect on mortality (HR 0.92, 95% CI 0.80 to 1.05, 10 studies) compared with conventional chemotherapy alone. Similarly, it may have little effect on progression-free survival when compared with conventional chemotherapy alone (HR 0.90, 95% CI 0.78 to 1.04, 11 studies; low-quality evidence). We did not find evidence from subgroup analysis that survival outcomes differed by type of molecular-targeted agent (EGFR- or VEGF-targeting agents) or tumor type, meaning that we were unable to explain the variation in effect across the studies by the presence or absence of prognostic biomarkers or type of molecular-targeted agent. From 11 eligible trials, we were able to use data from 3723 participants with measurable tumors. We found low-quality evidence that molecular-targeted therapy may increase tumor response (OR 1.24, 95% CI 1.00 to 1.55, low-quality evidence). Data from one small trial were too limited to determine the effect of treatment on quality of life (very low-quality evidence). The addition of targeted therapy to chemotherapy probably increases the risk of adverse events (OR 2.23, 95% CI 1.27 to 3.92, 5 trials, 2290 participants, moderate-quality evidence) and severe adverse event (OR 1.19, 95% CI 1.03 to 1.37, 8 trials, 3800 participants), compared with receiving chemotherapy alone.
Authors' conclusions: There is uncertainty about the effect of adding targeted therapy to chemotherapy on survival outcomes in people with advanced gastric cancer, with very little information on its impact on quality of life. There is more certain evidence of increased risk of adverse events and serious adverse events. The main limitation of the evidence for survival outcomes was inconsistency of effects across the studies, which we could not explain by prespecified subgroups in terms of the type of therapy or tumor type. Ongoing studies in this area are small and unlikely to improve our understanding of the effects of targeted therapy, and larger studies are needed.
Conflict of interest statement
Huan Song: none known
Jianwei Zhu: none known
DongHao Lu: none known
Figures
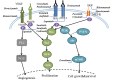













Update of
References
References to studies included in this review
Bang 2010 {published data only}
-
- Bang YJ, Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet 2010;376(9742):687‐97. - PubMed
-
- Ye M, Chen H, Shen JF. Costs of trastuzumab in combination with chemotherapy for HER2‐positive advanced gastric or gastroesophageal junction cancer: an economic evaluation in the Chinese context. Clinical Therapeutics 2012;34(2):468‐79. - PubMed
Eatock 2013 {published data only}
-
- Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Valladares‐Ayerbes M, Swieboda‐Sadlej A, et al. Phase II randomized, double‐blind, placebo‐controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro‐oesophageal cancer. Annals of Oncology 2013;24(3):710‐8. - PubMed
Hecht 2013 {published data only}
-
- Hecht JR, Bang Y, Qin S, Chung H, Xu J, Park J, et al. Lapatinib in combination with capecitabine plusoxaliplatin in HER2‐positive advanced ormetastatic gastric, esophageal, orgastroesophageal adenocarcinoma: the TRIO‐013/LOGiC trial. Journal of Clinical Oncology 2013;31(Suppl):abstr LBA4001.
Iveson 2014 {published data only}
-
- Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first‐line treatment for gastric or oesophagogastric junction adenocarcinoma: an open‐label, dose de‐escalation phase 1b study and a double‐blind, randomised phase 2study. Lancet Oncology 2014;15(9):1007‐18. - PubMed
Koizumi 2013 {published data only}
Lordick 2013 {published data only}
-
- Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open‐label phase 3 trial. Lancet Oncology 2013;14(6):490‐9. - PubMed
Ohtsu 2011 {published data only}
-
- Ohtsu A, Shah MA, Cutsem E, Rha SY, Sawaki A, Park SRl, et al. Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: a randomized, double‐blind, placebo‐controlled phase III study. Journal of Clinical Oncology 2011;29(30):3968‐76. - PubMed
-
- Shah M, Kang Y, Ohtsu A, Delmar P, Foernzler D, Langer B, et al. Tumor and blood plasma biomarker analyses in the avagast phase III randomized study of first line bevacizumab 1 capecitabine/cisplatin in patients with advanced gastric cancer. Annals of Oncology 2010;21:viii67‐viii68.
Rao 2010 {published data only}
-
- Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first‐line treatment in patients with advanced oesophago‐gastric cancer: a randomised, multicentre open‐label phase II study. Annals of Oncology 2010;21(11):2213‐9. - PubMed
Shen 2015 {published data only}
-
- Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double‐blind, phase III study (AVATAR study). Gastric Cancer 2015;18(1):168‐76. - PMC - PubMed
Waddell 2013 {published data only}
-
- Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open‐label phase 3 trial. Lancet Oncology 2013;14(6):481‐9. - PMC - PubMed
References to studies excluded from this review
Liang 2009 {published data only}
-
- Liang J, Liang S, Li G, Liu H, Zhao L, Wang H, et al. Clinical efficacy of rh‐endostatin combined with XELOX for advanced gastric cancer. [Chinese]. Chinese Journal of Clinical Oncology 2009;36(17):976‐9.
Ohtsu 2013 {published data only}
Qin 2014 {published data only}
-
- Qin SK. Phase III study of apatinib in advanced gastric cancer: A randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2014;32(5s):suppl; abstr 4003.
Richards 2013 {published data only}
-
- Richards D, Kocs DM, Spira AI, David McCollum A, Diab S, Hecker LI, et al. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomised Phase 2 study. European Journal of Cancer 2013;49(13):2823‐31. - PubMed
Satoh 2014 {published data only}
-
- Satoh T, Doi T, Ohtsu A, Tsuji A, Omuro Y, Mukaiyama A, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second‐line treatment of HER2‐amplified advanced gastric cancer in Asian populations: TyTAN ‐ A randomized, phase III study. Journal of Clinical Oncology 2014;32(19):2039‐49. - PubMed
Sun 2013 {published data only}
-
- Sun GP, Xu RH, Xu JM, Li J, Wang JW, Qin S, et al. The Chinese subgroup from a randomized phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone as second‐line treatment of HER2‐amplified advanced gastric cancer (AGC) in Asian countries. Journal of Clinical Oncology 2013;suppl:abstr 4109.
Takahashi 2014 {published data only}
-
- Takahashi T, Nishikawa K, Miki A, Noshiro H, Yoshikawa T, Nishida Y, et al. Efficacy and safety result of trastuzumab (T‐mab) and paclitaxel for T‐mab naive patients with HER2‐positive previously treated advanced or recurrent gastric cancer (JFMC45‐1102): Final report. Journal of Clinical Oncology 2014;32(Suppl 3):abstr 79. - PubMed
Xu 2013 {published data only}
-
- Xu R, Ma N, Wang F, Ma L, Chen R, Chen R, et al. Results of a randomized and controlled clinical trial evaluating the efficacy and safety of combination therapy with Endostar and S‐1 combined with oxaliplatin in advanced gastric cancer. Onco Targets and Therapy 2013;6:925‐9. [DOI: 10.2147/OTT.S46487] - DOI - PMC - PubMed
References to studies awaiting assessment
Wahab 2011 {published data only}
-
- Wahab MA, Ezzelarab L, Bendary S. Cetuximab plus capecitabine and oxaloplatin for chemonaive patients with advanced gastric cancer. Annals of Oncology 2011;22:v50.
Wang 2012 {published data only}
-
- Wang JW, Chi Y, Zheng ZX, Qu T, Zhou AP, Yang L, et al. Randomized, single‐centered, phase II clinical trial of nimotuzumab plus cisplatin and S‐1 as first‐line therapy in patients with advanced gastric cancer. Journal of Clinical Oncology 2012;30(Suppl; abstr):e14668.
References to ongoing studies
ACTRN12609000109202 {published data only}
-
- A randomised phase II study evaluating weekly docetaxel, cisplatin, fluoropyrimidine (wTCF) plus or minus panitumumab in advanced oesophago‐gastric cancer. Ongoing study March 2010.
NCT01123473 {published data only}
-
- Effectiveness of first line treatment with lapatinib and ECF/X in histologically proven adenocarcinoma of the stomach or the esophagogastric junction, metastatic or not amenable to curative surgery according to HER2 and EGFR status: a randomized Phase II trial. Ongoing study May 13 2010.
NCT01443065 {published data only}
-
- MEGA (Met or EGFR Inhibition in Gastroesophageal Adenocarcinoma): FOLFOX alone or in combination with AMG 102 or panitumumab as first‐line treatment in patients with advanced gastroesophageal adenocarcinoma. Ongoing study January 2011.
NCT01503372 {published data only}
-
- Pazopanib with 5‐Fluorouracil, leucovorin and oxaliplatin (FLO) as 1st‐line treatment in advanced gastric cancer; a randomized hase‐II‐study of the Arbeitsgemeinschaft Internistische Onkologie. Ongoing study November 2011.
NCT01662869 {published data only}
-
- A study of onartuzumab (MetMAb) in combination with mFOLFOX6 in patients with metastatic HER2‐negative and Met‐positive gastroesophageal cancer (MetGastric). Ongoing study November 2012.
NCT01774786 {published data only}
-
- A study of perjeta (Pertuzumab) in combination with herceptin (trastuzumab) and chemotherapy in patients with HER2‐positive metastatic gastroesophageal junction or gastric cancer. Ongoing study June 2013.
NCT02314117 {published data only}
-
- A randomized, double‐blind, placebo‐controlled Phase 3 study of capecitabine and cisplatin with or without ramucirumab as first‐line therapy in patients with metastatic gastric or gastroesophageal junction adenocarcinoma (RAINFALL). Ongoing study January 2015.
Additional references
Anderson 2010
Bang 2012
-
- Bang YJ. Advances in the management of HER2‐positive advanced gastric and gastroesophageal junction cancer. Journal of Clinical Gastroenterology 2012;46(8):637‐48. - PubMed
Begnami 2011
-
- Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, Costa WL Jr, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. Journal of Clinical Oncology 2011;29(22):3030‐6. - PubMed
Borenstein 2008
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Anaysis (Statistics in Practice). Chichester (UK): John Wiley & Sons, 2008.
Carmeliet 2003
-
- Carmeliet P. Angiogenesis in health and disease. Nature Medicine 2003;9(6):653‐60. - PubMed
Chua 2011
-
- Chua TC, Merrett ND. Clinicopathologic factors associated with HER2‐positive gastric cancer and its impact on survival outcomes‐‐a systematic review. International Journal of Cancer 2012;130(12):2845‐56. - PubMed
Draovich 2006
-
- Dragovich T, McCoy S, Fenoglio‐Preiser CM, Wang J, Benedetti JK, Baker AF, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. Journal of Clinical Oncology 2006;24(30):4922‐7. - PubMed
European Medicines Agency 2013
-
- European Medicines Agency. Herceptin 150 mg powder for concentrate for solution for infusion: summary of product characteristics. www.emea.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Informatio.... (Accessed 6 November 2013).
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns inGLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. - PubMed
Genentech 2013
-
- Genentech Inc. Herceptin (trastuzumab): US prescribing information 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5256lbl.pdf. (Accessed 6 November 2013).
Grabsch 2010
GRADEprofiler 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEprofiler (GRADEpro). Version 3.2. GRADE Working Group, 2008.
Graziano 2011
-
- Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D'Emidio S, et al. Genetic activation of the MET pathway and prognosis of patients with high‐risk, radically resected gastric cancer. Journal of Clinical Oncology 2011;29(36):4789‐95. - PubMed
Hanahan 1996
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86(3):353‐64. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ilson 2011
Iqbal 2011
Kim 2008
-
- Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 2008;52(6):738‐46. - PubMed
Kim 2011
-
- Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole‐tissue sections and 595 cases of tissue microarrays. Annals of Surgical Oncology 2011;18(10):2833‐40. - PubMed
Kim 2013
Lieto 2008
-
- Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Annals of Surgical Oncology 2008;15(1):69‐79. - PubMed
Lordick 2010b
-
- Lordick F. Trastuzumab: a new treatment option for HER2‐positive metastatic gastric and gastroesophageal junction cancer. Future Oncology 2011;7(2):187‐99. - PubMed
Macdonald 2001
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New England Journal of Medicine 2001;345(10):725‐30. - PubMed
Maeda 1996
-
- Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77(5):858‐63. - PubMed
Murayama 2009
Ni 2010
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Pohl 2005
-
- Pohl H, Welch HG. The role of over diagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute 2005;97(2):142‐6. - PubMed
Power 2010
-
- Power DG, Kelsen DP, Shah MA. Advanced gastric cancer‐‐slow but steady progress. Cancer Treatment Reviews 2010;36(5):384‐92. - PubMed
Price 2012
-
- Price TJ, Shapiro JD, Segelov E, Karapetis CS, Pavlakis N, Cutsem E, et al. Management of advanced gastric cancer. Expert Review of Gastroenterology & Hepatology 2012;6(2):199‐208. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2013.
Sakuramoto 2007
-
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. New England Journal of Medicine 2007;357(18):1810‐20. - PubMed
Shaw 2006
-
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441(7092):424‐30. - PubMed
Song 2015a
-
- Song H, Held M, Sandin S, Rautelin H, Eliasson M, Söderberg S, et al. Increase in the prevalence of atrophic gastritis among adults age 35 to 44 years old in Northern Sweden between 1990 and 2009. Clinical Gastroenterology and Hepatology 2015;13(9):1592‐600. - PubMed
Spector 2009
-
- Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2‐positive breast cancer. Journal of Clinical Oncology 2009;27(34):5838‐47. - PubMed
Sun 2010
-
- Sun W, Powell M, O'Dwyer PJ, Catalano P, Ansari RH, Benson AB 3rd. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. Journal of Clinical Oncology 2010;28(18):2947‐51. - PMC - PubMed
Tanigawa 1996
-
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Research 1996;56(11):2671‐6. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, National Cancer Institute of the United States. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Tierney 2007
Wagner 2006
-
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta‐analysis based on aggregate data. Journal of Clinical Oncology 2006;24(18):2903‐9. - PubMed
Wagner 2009
-
- Wagner AD, Moehler M. Development of targeted therapies in advanced gastric cancer: promising exploratory steps in a new era. Current Opinion in Oncology 2009;21(4):381‐5. - PubMed
Wagner 2010
Widakowich 2007
-
- Widakowich C, Castro G Jr, Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist 2007;12(12):1443‐55. - PubMed
Wilhelm 2008
-
- Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Molecular Cancer Therapeutics 2008;7(10):3129‐40. - PubMed
Wu 2009
-
- Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. Journal of Gastroenterology and Hepatology 2009;24(1):37‐41. - PubMed
Yano 2006
-
- Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncology Reports 2006;15(1):65‐71. - PubMed
Yu 2009
-
- Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clinical Cancer Research 2009;15(5):1821‐9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

