Investigation of MicroRNA-21 Expression Levels in Serum and Stool as a Potential Non-Invasive Biomarker for Diagnosis of Colorectal Cancer
- PMID: 27432735
- PMCID: PMC5274709
- DOI: 10.18869/acadpub.ibj.21.2.106
Investigation of MicroRNA-21 Expression Levels in Serum and Stool as a Potential Non-Invasive Biomarker for Diagnosis of Colorectal Cancer
Abstract
Background: Most cancer studies focus on exploring non-invasive biomarkers for cancer detection. In the present study, we sought to investigate the expression level of microRNA-21 (miR-21), as a potential diagnostic marker, in serum and stool samples from 40 patients with colorectal cancer (CRC) and 40 healthy controls.
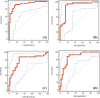
Methods: Quantitative real-time RT-PCR was applied to determine the relative expression level of miR-21 in serum and stool. At the same time, the sensitivity and specificity of this marker was evaluated by receiver operating characteristic (ROC) curve analysis.
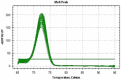
Results: miR-21 expression levels of serum and stool were up-regulated 12.1 (P<0.05, 95% CI: 5.774-34.045) and 10.0 (P<0.05, 95% CI: 0.351-16.260) times in CRC patients, respectively, when compared to the control group. The sensitivity and specificity of miR-21 was found to be 86.05% and 72.97%, respectively (an area under the ROC curve [AUC] of 0.783). The stool miR-21 level in CRC patients was much higher than that in the healthy controls, showing a sensitivity of 86.05% and a specificity of 81.08% (AUC: 0.829). The expression level of miR-21 in stool was able to significantly distinguish CRC tumor, node, metastasis stages III-IV from stages I-II, with a sensitivity and specificity of 88.1% and 81.6%, respectively (AUC: 0.872).
Conclusion: The results of this study indicated that miR-21 expression levels in serum and stool can be considered as a potential diagnostic biomarker for the diagnosis of CRC patients. However, more studies are required to confirm the validity of miR-21 as a valuable non-invasive diagnostic tool for CRC.
Keywords: Serum; Stool; miR-21; Biomarker; Colorectal Cancer (CRC).
Conflict of interest statement
CONFLICT OF INTEREST. None declared.
Figures

Similar articles
-
Identification of stool miR-135b-5p as a non-invasive diaognostic biomarker in later tumor stage of colorectal cancer.Life Sci. 2020 Nov 1;260:118417. doi: 10.1016/j.lfs.2020.118417. Epub 2020 Sep 12. Life Sci. 2020. PMID: 32931801
-
Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer.Tumour Biol. 2015 Mar;36(3):1619-25. doi: 10.1007/s13277-014-2760-9. Epub 2014 Dec 21. Tumour Biol. 2015. PMID: 25528214
-
Serum miR-663 expression and the diagnostic value in colorectal cancer.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):2650-2653. doi: 10.1080/21691401.2019.1628036. Artif Cells Nanomed Biotechnol. 2019. PMID: 31240955
-
Diagnostic performance of microRNA-29a for colorectal cancer: a meta-analysis.Genet Mol Res. 2015 Dec 22;14(4):18018-25. doi: 10.4238/2015.December.22.28. Genet Mol Res. 2015. PMID: 26782449
-
Diagnostic value of circulating miR-21 for colorectal cancer: a meta-analysis.Cancer Biomark. 2015;15(1):47-56. doi: 10.3233/CBM-140437. Cancer Biomark. 2015. PMID: 25524942 Review.
Cited by
-
Liquid biopsy approaches and immunotherapy in colorectal cancer for precision medicine: Are we there yet?Front Oncol. 2023 Jan 6;12:1023565. doi: 10.3389/fonc.2022.1023565. eCollection 2022. Front Oncol. 2023. PMID: 36686736 Free PMC article. Review.
-
Fecal microRNAs as Innovative Biomarkers of Intestinal Diseases and Effective Players in Host-Microbiome Interactions.Cancers (Basel). 2020 Aug 5;12(8):2174. doi: 10.3390/cancers12082174. Cancers (Basel). 2020. PMID: 32764361 Free PMC article. Review.
-
Evaluation of Tissue and Circulating miR-21 as Potential Biomarker of Response to Chemoradiotherapy in Rectal Cancer.Pharmaceuticals (Basel). 2020 Sep 14;13(9):246. doi: 10.3390/ph13090246. Pharmaceuticals (Basel). 2020. PMID: 32937907 Free PMC article.
-
The diagnostic value of serum miR-21 in patients with ovarian cancer: a systematic review and meta-analysis.J Ovarian Res. 2022 May 2;15(1):51. doi: 10.1186/s13048-022-00985-3. J Ovarian Res. 2022. PMID: 35501921 Free PMC article.
-
Detecting colorectal cancer using genetic and epigenetic biomarkers: screening and diagnosis.J Med Life. 2024 Jan;17(1):4-14. doi: 10.25122/jml-2023-0269. J Med Life. 2024. PMID: 38737656 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. - PubMed
-
- Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics 2014. CA: a cancer journal for clinicians. 2014;64(2):104–117. - PubMed
-
- Bresalier RS, Kopetz S, Brenner DE. Blood-based tests for colorectal cancer screening: do they threaten the survival of the FIT test? Digestive diseases and sciences. 2015;60(3):664–671. - PubMed
-
- Leggett BA, Hewett DG. Colorectal cancer screening. Internal medicine journal. 2015;45:6–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
