An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria
- PMID: 27432743
- PMCID: PMC5280024
- DOI: 10.1681/ASN.2016030338
An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria
Abstract
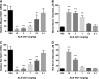
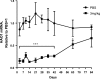
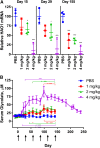
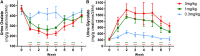
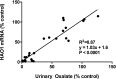
Primary hyperoxaluria type 1 (PH1), an inherited rare disease of glyoxylate metabolism, arises from mutations in the enzyme alanine-glyoxylate aminotransferase. The resulting deficiency in this enzyme leads to abnormally high oxalate production resulting in calcium oxalate crystal formation and deposition in the kidney and many other tissues, with systemic oxalosis and ESRD being a common outcome. Although a small subset of patients manages the disease with vitamin B6 treatments, the only effective treatment for most is a combined liver-kidney transplant, which requires life-long immune suppression and carries significant mortality risk. In this report, we discuss the development of ALN-GO1, an investigational RNA interference (RNAi) therapeutic targeting glycolate oxidase, to deplete the substrate for oxalate synthesis. Subcutaneous administration of ALN-GO1 resulted in potent, dose-dependent, and durable silencing of the mRNA encoding glycolate oxidase and increased serum glycolate concentrations in wild-type mice, rats, and nonhuman primates. ALN-GO1 also increased urinary glycolate concentrations in normal nonhuman primates and in a genetic mouse model of PH1. Notably, ALN-GO1 reduced urinary oxalate concentration up to 50% after a single dose in the genetic mouse model of PH1, and up to 98% after multiple doses in a rat model of hyperoxaluria. These data demonstrate the ability of ALN-GO1 to reduce oxalate production in preclinical models of PH1 across multiple species and provide a clear rationale for clinical trials with this compound.
Keywords: RNAi therapeutics; end-stage renal disease; oxalate; primary hyperoxaluria type I; siRNA.
Copyright © 2017 by the American Society of Nephrology.
Figures

Comment in
-
Stones: A novel RNAi therapy for PH1.Nat Rev Nephrol. 2016 Sep;12(9):508. doi: 10.1038/nrneph.2016.123. Epub 2016 Aug 8. Nat Rev Nephrol. 2016. PMID: 27498954 No abstract available.
-
Re: An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria.J Urol. 2017 May;197(5):1297-1298. doi: 10.1016/j.juro.2017.02.010. Epub 2017 Feb 9. J Urol. 2017. PMID: 29539912 No abstract available.
Similar articles
-
Inhibition of Glycolate Oxidase With Dicer-substrate siRNA Reduces Calcium Oxalate Deposition in a Mouse Model of Primary Hyperoxaluria Type 1.Mol Ther. 2016 Apr;24(4):770-8. doi: 10.1038/mt.2016.4. Epub 2016 Jan 13. Mol Ther. 2016. PMID: 26758691 Free PMC article.
-
Metabolism of (13)C5-hydroxyproline in mouse models of Primary Hyperoxaluria and its inhibition by RNAi therapeutics targeting liver glycolate oxidase and hydroxyproline dehydrogenase.Biochim Biophys Acta. 2016 Feb;1862(2):233-9. doi: 10.1016/j.bbadis.2015.12.001. Epub 2015 Dec 2. Biochim Biophys Acta. 2016. PMID: 26655602 Free PMC article.
-
Systemic Alanine Glyoxylate Aminotransferase mRNA Improves Glyoxylate Metabolism in a Mouse Model of Primary Hyperoxaluria Type 1.Nucleic Acid Ther. 2019 Apr;29(2):104-113. doi: 10.1089/nat.2018.0740. Epub 2019 Jan 24. Nucleic Acid Ther. 2019. PMID: 30676254
-
Human glyoxylate metabolism revisited: New insights pointing to multi-organ involvement with implications for siRNA-based therapies in primary hyperoxaluria.J Inherit Metab Dis. 2025 Jan;48(1):e12817. doi: 10.1002/jimd.12817. Epub 2024 Nov 24. J Inherit Metab Dis. 2025. PMID: 39582099 Free PMC article. Review.
-
New therapeutics for primary hyperoxaluria type 1.Curr Opin Nephrol Hypertens. 2022 Jul 1;31(4):344-350. doi: 10.1097/MNH.0000000000000790. Epub 2022 Mar 9. Curr Opin Nephrol Hypertens. 2022. PMID: 35266883 Free PMC article. Review.
Cited by
-
Ligand conjugate SAR and enhanced delivery in NHP.Mol Ther. 2021 Oct 6;29(10):2910-2919. doi: 10.1016/j.ymthe.2021.06.002. Epub 2021 Jun 4. Mol Ther. 2021. PMID: 34091052 Free PMC article.
-
Stones: A novel RNAi therapy for PH1.Nat Rev Nephrol. 2016 Sep;12(9):508. doi: 10.1038/nrneph.2016.123. Epub 2016 Aug 8. Nat Rev Nephrol. 2016. PMID: 27498954 No abstract available.
-
Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria.Br J Clin Pharmacol. 2022 Jun;88(6):2525-2538. doi: 10.1111/bcp.14925. Epub 2021 Jun 11. Br J Clin Pharmacol. 2022. PMID: 34022071 Free PMC article. Review.
-
A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights.Lab Anim Res. 2024 Apr 22;40(1):17. doi: 10.1186/s42826-024-00195-6. Lab Anim Res. 2024. PMID: 38649954 Free PMC article. Review.
-
Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs.Biomolecules. 2024 Apr 23;14(5):511. doi: 10.3390/biom14050511. Biomolecules. 2024. PMID: 38785918 Free PMC article. Review.
References
-
- Hoppe B: An update on primary hyperoxaluria. Nat Rev Nephrol 8: 467–475, 2012 - PubMed
-
- Kamoun A, Lakhoua R: End-stage renal disease of the Tunisian child: epidemiology, etiologies, and outcome. Pediatr Nephrol 10: 479–482, 1996 - PubMed
-
- Al-Eisa AA, Samhan M, Naseef M: End-stage renal disease in Kuwaiti children: an 8-year experience. Transplant Proc 36: 1788–1791, 2004 - PubMed
-
- Boualla L, Tajir M, Oulahiane N, Lyahyai J, Laarabi FZ, Chafai Elalaoui S, Soulami K, Ait Ouamar H, Sefiani A: AGXT Gene Mutations and Prevalence of Primary Hyperoxaluria Type 1 in Moroccan Population. Genet Test Mol Biomarkers 19: 623–628, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

