The WASF3-NCKAP1-CYFIP1 Complex Is Essential for Breast Cancer Metastasis
- PMID: 27432794
- PMCID: PMC5010469
- DOI: 10.1158/0008-5472.CAN-16-0562
The WASF3-NCKAP1-CYFIP1 Complex Is Essential for Breast Cancer Metastasis
Abstract
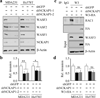
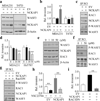
Inactivation of the WASF3 gene suppresses invasion and metastasis of breast cancer cells. WASF3 function is regulated through a protein complex that includes the NCKAP1 and CYFIP1 proteins. Here, we report that silencing NCKAP1 destabilizes the WASF3 complex, resulting in a suppression of the invasive capacity of breast, prostate, and colon cancer cells. In an in vivo model of spontaneous metastasis in immunocompromized mice, loss of NCKAP1 also suppresses metastasis. Activation of the WASF protein complex occurs through interaction with RAC1, and inactivation of NCKAP1 prevents the association of RAC1 with the WASF3 complex. Thus, WASF3 depends on NCKAP1 to promote invasion and metastasis. Here, we show that stapled peptides targeting the interface between NCKAP1 and CYFIP1 destabilize the WASF3 complex and suppress RAC1 binding, thereby suppressing invasion. Using a complex-disrupting compound identified in this study termed WANT3, our results offer a mechanistic proof of concept to target this interaction as a novel approach to inhibit breast cancer metastasis. Cancer Res; 76(17); 5133-42. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
of Potential Conflicts of Interest The authors declare no competing financial interests.
Figures

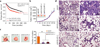
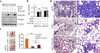
Similar articles
-
Suppression of Breast Cancer Metastasis Using Stapled Peptides Targeting the WASF Regulatory Complex.Cancer Growth Metastasis. 2017 Jun 19;10:1179064417713197. doi: 10.1177/1179064417713197. eCollection 2017. Cancer Growth Metastasis. 2017. PMID: 28680267 Free PMC article.
-
Targeting the WASF3-CYFIP1 Complex Using Stapled Peptides Suppresses Cancer Cell Invasion.Cancer Res. 2016 Feb 15;76(4):965-73. doi: 10.1158/0008-5472.CAN-15-1680. Epub 2015 Dec 16. Cancer Res. 2016. PMID: 26676744 Free PMC article.
-
Targeting the WASF3 complex to suppress metastasis.Pharmacol Res. 2022 Aug;182:106302. doi: 10.1016/j.phrs.2022.106302. Epub 2022 Jun 9. Pharmacol Res. 2022. PMID: 35691539 Review.
-
Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein.Oncogene. 2016 Jan 21;35(3):333-43. doi: 10.1038/onc.2015.86. Epub 2015 Mar 30. Oncogene. 2016. PMID: 25823022 Free PMC article.
-
Targeting WASF3 Signaling in Metastatic Cancer.Int J Mol Sci. 2021 Jan 15;22(2):836. doi: 10.3390/ijms22020836. Int J Mol Sci. 2021. PMID: 33467681 Free PMC article. Review.
Cited by
-
Two-Dimensional-PAGE Coupled with nLC-MS/MS-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in MCF7 Breast Cancer Cells Transfected for JTB Protein Silencing.Molecules. 2023 Nov 9;28(22):7501. doi: 10.3390/molecules28227501. Molecules. 2023. PMID: 38005222 Free PMC article.
-
SHOX2 cooperates with STAT3 to promote breast cancer metastasis through the transcriptional activation of WASF3.J Exp Clin Cancer Res. 2021 Aug 31;40(1):274. doi: 10.1186/s13046-021-02083-6. J Exp Clin Cancer Res. 2021. PMID: 34465361 Free PMC article.
-
Suppression of Breast Cancer Metastasis Using Stapled Peptides Targeting the WASF Regulatory Complex.Cancer Growth Metastasis. 2017 Jun 19;10:1179064417713197. doi: 10.1177/1179064417713197. eCollection 2017. Cancer Growth Metastasis. 2017. PMID: 28680267 Free PMC article.
-
Disulfidptosis, A Novel Cell Death Pathway: Molecular Landscape and Therapeutic Implications.Aging Dis. 2024 May 2;16(2):917-945. doi: 10.14336/AD.2024.0083. Aging Dis. 2024. PMID: 38739940 Free PMC article. Review.
-
Integrated machine learning-driven disulfidptosis profiling: CYFIP1 and EMILIN1 as therapeutic nodes in neuroblastoma.J Cancer Res Clin Oncol. 2024 Mar 1;150(3):109. doi: 10.1007/s00432-024-05630-8. J Cancer Res Clin Oncol. 2024. PMID: 38427078 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The next generation. Cell. 2011;144:646–674. - PubMed
-
- Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–823. - PubMed
-
- Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. - PubMed
-
- Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:21748–21755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

