Tract-specific white matter hyperintensities disrupt neural network function in Alzheimer's disease
- PMID: 27432800
- PMCID: PMC5319922
- DOI: 10.1016/j.jalz.2016.06.2358
Tract-specific white matter hyperintensities disrupt neural network function in Alzheimer's disease
Abstract
Introduction: White matter hyperintensities (WMHs) increase the risk of Alzheimer's disease (AD). Whether WMHs are associated with the decline of functional neural networks in AD is debated.
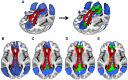
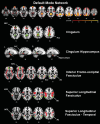
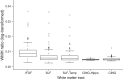
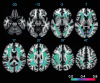
Method: Resting-state functional magnetic resonance imaging and WMH were assessed in 78 subjects with increased amyloid levels on AV-45 positron emission tomography (PET) in different clinical stages of AD. We tested the association between WMH volume in major atlas-based fiber tract regions of interest (ROIs) and changes in functional connectivity (FC) between the tracts' projection areas within the default mode network (DMN).
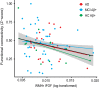
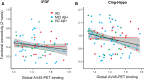
Results: WMH volume within the inferior fronto-occipital fasciculus (IFOF) was the highest among all tract ROIs and associated with reduced FC in IFOF-connected DMN areas, independently of global AV-45 PET. Higher AV-45 PET contributed to reduced FC in IFOF-connected, temporal, and parietal DMN areas.
Conclusions: High fiber tract WMH burden is associated with reduced FC in connected areas, thus adding to the effects of amyloid pathology on neuronal network function.
Keywords: Alzheimer's disease; Amyloid-beta; Fiber tract; Functional connectivity; Resting-state fMRI; Vascular; White matter hyperintensities.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Differential Effects of Confluent and Nonconfluent White Matter Hyperintensities on Functional Connectivity in Mild Cognitive Impairment.Brain Connect. 2020 Dec;10(10):547-554. doi: 10.1089/brain.2020.0784. Epub 2020 Nov 16. Brain Connect. 2020. PMID: 33050714
-
Age and Alzheimer's pathology disrupt default mode network functioning via alterations in white matter microstructure but not hyperintensities.Cortex. 2018 Jul;104:58-74. doi: 10.1016/j.cortex.2018.04.006. Epub 2018 Apr 19. Cortex. 2018. PMID: 29758374 Free PMC article.
-
Spatial-temporal interactions between white matter hyperintensities and multiple pathologies across the Alzheimer's disease continuum.Alzheimers Dement. 2025 Apr;21(4):e70098. doi: 10.1002/alz.70098. Alzheimers Dement. 2025. PMID: 40302045 Free PMC article.
-
Measuring Cortical Connectivity in Alzheimer's Disease as a Brain Neural Network Pathology: Toward Clinical Applications.J Int Neuropsychol Soc. 2016 Feb;22(2):138-63. doi: 10.1017/S1355617715000995. J Int Neuropsychol Soc. 2016. PMID: 26888613 Review.
-
White matter hyperintensities as early and independent predictors of Alzheimer's disease risk.J Alzheimers Dis. 2014;42 Suppl 4:S393-400. doi: 10.3233/JAD-141473. J Alzheimers Dis. 2014. PMID: 25261452 Review.
Cited by
-
Extreme capsule is a bottleneck for ventral pathway.IBRO Neurosci Rep. 2021 Feb 3;10:42-50. doi: 10.1016/j.ibneur.2020.11.002. eCollection 2021 Jun. IBRO Neurosci Rep. 2021. PMID: 33861816 Free PMC article. Review.
-
Aberrant White Matter Microstructure as a Potential Diagnostic Marker in Alzheimer's Disease by Automated Fiber Quantification.Front Neurosci. 2020 Sep 24;14:570123. doi: 10.3389/fnins.2020.570123. eCollection 2020. Front Neurosci. 2020. PMID: 33071742 Free PMC article.
-
Metabolic Messengers: ketone bodies.Nat Metab. 2023 Dec;5(12):2062-2074. doi: 10.1038/s42255-023-00935-3. Epub 2023 Dec 13. Nat Metab. 2023. PMID: 38092961 Review.
-
Letter.Alzheimers Dement (Amst). 2017 Nov 21;9:84-87. doi: 10.1016/j.dadm.2017.11.004. eCollection 2017. Alzheimers Dement (Amst). 2017. PMID: 29255790 Free PMC article. No abstract available.
-
Damage to white matter networks resulting from small vessel disease and the effects on cognitive function.Sci Rep. 2025 Jul 30;15(1):27736. doi: 10.1038/s41598-025-13813-7. Sci Rep. 2025. PMID: 40731161 Free PMC article.
References
-
- Reed B.R., Eberling J.L., Mungas D., Weiner M., Kramer J.H., Jagust W.J. Effects of white matter lesions and lacunes on cortical function. Arch Neurol. 2004;61:1545–1550. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

