Safety Profile of Nifurtimox for Treatment of Chagas Disease in the United States
- PMID: 27432838
- PMCID: PMC5036918
- DOI: 10.1093/cid/ciw477
Safety Profile of Nifurtimox for Treatment of Chagas Disease in the United States
Abstract
Background: Nifurtimox is 1 of only 2 medications available for treating Chagas disease (CD) and currently the only drug available in the United States, but its safety and tolerance have not been extensively studied. This is the first study to evaluate tolerance of nifurtimox in US patients with CD.
Methods: This investigation assessed side effects in a sample of 53 patients with CD, all Latin American immigrants, who underwent treatment with nifurtimox (8-10 mg/kg in 3 daily doses for 12 weeks) from March 2008 to July 2012. The frequency and severity of adverse events (AEs) was recorded.
Results: A total of 435 AEs were recorded; 93.8% were mild, 3.0% moderate, and 3.2% severe. Patients experienced a mean of 8.2 AEs; the most frequent were anorexia (79.2%), nausea (75.5%), headache (60.4%), amnesia (58.5%), and >5% weight loss (52.8%). Eleven patients (20.8%) were unable to complete treatment. Experiencing a moderate or severe AE (odds ratio [OR], 3.82; P < .05) and Mexican nationality (OR, 2.29; P < .05) were significant predictors of treatment discontinuation, but sex and cardiac progression at baseline were not. Patients who did not complete treatment experienced nearly 3 times more AEs per 30-day period (P = .05).
Conclusions: Nifurtimox produces frequent side effects, but the majority are mild and can be managed with dose reduction and/or temporary suspension of medication. The high frequency of gastrointestinal symptoms and weight loss mirrors results from prior investigations. Special attention should be paid during the early stages of treatment to potentially severe symptoms including depression, rash, and anxiety.
Keywords: Chagas disease; Trypanosoma cruzi; benznidazole; nifurtimox; side effects.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

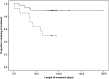
 = Patients moving toward premature termination.
= Patients moving toward premature termination.  = Patients moving toward treatment completion.
= Patients moving toward treatment completion.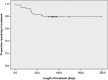
References
-
- World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Geneva, Switzerland: WHO, 2015. - PubMed
-
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 2010; 115:14–21. - PubMed
-
- Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 2009; 49:e52–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

