Small-Conductance Ca2+-Activated Potassium Channels Negatively Regulate Aldosterone Secretion in Human Adrenocortical Cells
- PMID: 27432863
- PMCID: PMC4982792
- DOI: 10.1161/HYPERTENSIONAHA.116.07094
Small-Conductance Ca2+-Activated Potassium Channels Negatively Regulate Aldosterone Secretion in Human Adrenocortical Cells
Abstract
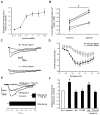
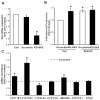
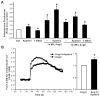
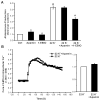
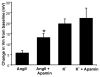
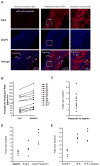
Aldosterone, which plays a key role in maintaining water and electrolyte balance, is produced by zona glomerulosa cells of the adrenal cortex. Autonomous overproduction of aldosterone from zona glomerulosa cells causes primary hyperaldosteronism. Recent clinical studies have highlighted the pathological role of the KCNJ5 potassium channel in primary hyperaldosteronism. Our objective was to determine whether small-conductance Ca(2+)-activated potassium (SK) channels may also regulate aldosterone secretion in human adrenocortical cells. We found that apamin, the prototypic inhibitor of SK channels, decreased membrane voltage, raised intracellular Ca(2+) and dose dependently increased aldosterone secretion from human adrenocortical H295R cells. By contrast, 1-Ethyl-2-benzimidazolinone, an agonist of SK channels, antagonized apamin's action and decreased aldosterone secretion. Commensurate with an increase in aldosterone production, apamin increased mRNA expression of steroidogenic acute regulatory protein and aldosterone synthase that control the early and late rate-limiting steps in aldosterone biosynthesis, respectively. In addition, apamin increased angiotensin II-stimulated aldosterone secretion, whereas 1-Ethyl-2-benzimidazolinone suppressed both angiotensin II- and high K(+)-stimulated production of aldosterone in H295R cells. These findings were supported by apamin-modulation of basal and angiotensin II-stimulated aldosterone secretion from acutely prepared slices of human adrenals. We conclude that SK channel activity negatively regulates aldosterone secretion in human adrenocortical cells. Genetic association studies are necessary to determine whether mutations in SK channel subtype 2 genes may also drive aldosterone excess in primary hyperaldosteronism.
Keywords: adrenal cortex aldosterone secretion; angiotensin II; apamin; hyperaldosteronism; small-conductance Ca2+-activated potassium channels.
© 2016 American Heart Association, Inc.
Figures

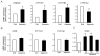
Comment in
-
Small Potassium Channels: Speculation on a Role to Regulate Aldosterone Production and Blood Pressure.Hypertension. 2016 Sep;68(3):542-3. doi: 10.1161/HYPERTENSIONAHA.116.07938. Epub 2016 Jul 18. Hypertension. 2016. PMID: 27432871 Free PMC article. No abstract available.
Similar articles
-
Effects of K+ channel blockers on K+ channels, membrane potential, and aldosterone secretion in rat adrenal zona glomerulosa cells.Endocrinology. 1997 Oct;138(10):4167-75. doi: 10.1210/endo.138.10.5463. Endocrinology. 1997. PMID: 9322926
-
Guanylin: a novel regulatory peptide possibly involved in the control of Ca2+-dependent agonist-stimulated aldosterone secretion in rats.Int J Mol Med. 1999 Jan;3(1):59-62. doi: 10.3892/ijmm.3.1.59. Int J Mol Med. 1999. PMID: 9864386
-
TASK1 and TASK3 potassium channels: determinants of aldosterone secretion and adrenocortical zonation.Horm Metab Res. 2010 Jun;42(6):450-7. doi: 10.1055/s-0029-1243601. Epub 2010 Jan 4. Horm Metab Res. 2010. PMID: 20049674 Review.
-
Blockade of T-type voltage-dependent Ca2+ channels by benidipine, a dihydropyridine calcium channel blocker, inhibits aldosterone production in human adrenocortical cell line NCI-H295R.Eur J Pharmacol. 2008 Apr 28;584(2-3):424-34. doi: 10.1016/j.ejphar.2008.02.001. Epub 2008 Feb 12. Eur J Pharmacol. 2008. PMID: 18331727
-
Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy?Endocrinology. 2014 Jan;155(1):47-55. doi: 10.1210/en.2013-1733. Epub 2013 Dec 20. Endocrinology. 2014. PMID: 24248457 Free PMC article. Review.
Cited by
-
Ion Channel Function and Electrical Excitability in the Zona Glomerulosa: A Network Perspective on Aldosterone Regulation.Annu Rev Physiol. 2021 Feb 10;83:451-475. doi: 10.1146/annurev-physiol-030220-113038. Epub 2020 Nov 11. Annu Rev Physiol. 2021. PMID: 33176563 Free PMC article. Review.
-
Small-conductance Ca2+-activated K+ channels: insights into their roles in cardiovascular disease.Exp Mol Med. 2018 Apr 13;50(4):1-7. doi: 10.1038/s12276-018-0043-z. Exp Mol Med. 2018. PMID: 29651007 Free PMC article. Review.
-
T- and L-Type Calcium Channels Maintain Calcium Oscillations in the Murine Zona Glomerulosa.Hypertension. 2024 Apr;81(4):811-822. doi: 10.1161/HYPERTENSIONAHA.123.21798. Epub 2024 Feb 16. Hypertension. 2024. PMID: 38507511 Free PMC article.
-
The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications.Endocr Rev. 2018 Dec 1;39(6):1029-1056. doi: 10.1210/er.2018-00060. Endocr Rev. 2018. PMID: 30007283 Free PMC article. Review.
-
Glucocorticoids modulate neural activity via a rapid non-genomic effect on Kv2.2 channels in the central nervous system.Neurobiol Stress. 2023 Nov 25;28:100593. doi: 10.1016/j.ynstr.2023.100593. eCollection 2024 Jan. Neurobiol Stress. 2023. PMID: 38075025 Free PMC article.
References
-
- Aguilera G, Catt KJ. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin ii and potassium. Endocrinology. 1986;118:112–118. - PubMed
-
- Capponi AM, Lew PD, Jornot L, Vallotton MB. Correlation between cytosolic free ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin ii and potassium. J Biol Chem. 1984;259:8863–8869. - PubMed
-
- Fakunding JL, Catt KJ. Dependence of aldosterone stimulation in adrenal glomerulosa cells on calcium uptake: Effects of lanthanum nd verapamil. Endocrinology. 1980;107:1345–1353. - PubMed
-
- Hausdorff WP, Aguilera G, Catt KJ. Selective enhancement of angiotensin ii- and potassium-stimulated aldosterone secretion by the calcium channel agonist bay k 8644. Endocrinology. 1986;118:869–874. - PubMed
-
- Kojima K, Kojima I, Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984;247:E645–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

