Protective Role of Kallistatin in Vascular and Organ Injury
- PMID: 27432868
- PMCID: PMC4982811
- DOI: 10.1161/HYPERTENSIONAHA.116.07861
Protective Role of Kallistatin in Vascular and Organ Injury
Abstract
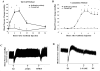
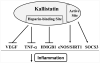
Kallistatin is an endogenous protein that exerts pleiotropic effects, including vasodilation and inhibition of angiogenesis, inflammation, oxidative stress, apoptosis, fibrosis, and tumor progression. Through its two functional domains – an active site and a heparin-binding site – kallistatin regulates differential signaling pathways and a wide spectrum of biological functions. Kallistatin's active site is key for inhibiting tissue kallikrein activity, and stimulating the expression of endothelial nitric oxide synthase (eNOS), sirtuin 1 (SIRT1) and suppressor of cytokine signaling 3 (SOCS3). Kallistatin via its heparin-binding site blocks signaling pathways mediated by growth factors and cytokines, such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), high mobility group box-1 (HMGB1), Wnt, transforming growth factor-β (TGF-β), and epidermal growth factor (EGF). Kallistatin gene or protein delivery protects against the pathogenesis of hypertension, heart and kidney damage, arthritis, sepsis, influenza virus infection, tumor growth and metastasis in animal models. Conversely, depletion of endogenous kallistatin by neutralizing antibody injection exacerbates cardiovascular and renal injury in hypertensive rats. Kallistatin levels are markedly reduced in rodents with hypertension, sepsis, streptozotocin-induced diabetes, and cardiac and renal injury. Kallistatin levels are also diminished in patients with liver disease, septic syndrome, diabetic retinopathy, severe pneumonia, inflammatory bowel disease, and obesity, prostate and colon cancer. Therefore, circulating kallistatin levels may serve as a new biomarker for human diseases. This review summarizes kallistatin's protective roles and mechanisms in vascular and organ injury, and highlights the therapeutic potential of kallistatin for multiple disease states.
Figures


Similar articles
-
Protective Role of Endogenous Kallistatin in Vascular Injury and Senescence by Inhibiting Oxidative Stress and Inflammation.Oxid Med Cell Longev. 2018 Dec 2;2018:4138560. doi: 10.1155/2018/4138560. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30622668 Free PMC article. Review.
-
Kallistatin is a potent new vasodilator.J Clin Invest. 1997 Jul 1;100(1):11-7. doi: 10.1172/JCI119502. J Clin Invest. 1997. PMID: 9202051 Free PMC article.
-
Kallistatin stimulates vascular smooth muscle cell proliferation and migration in vitro and neointima formation in balloon-injured rat artery.Circ Res. 2000 Mar 3;86(4):418-24. doi: 10.1161/01.res.86.4.418. Circ Res. 2000. PMID: 10700446
-
The Potential Role of Kallistatin in the Development of Abdominal Aortic Aneurysm.Int J Mol Sci. 2016 Aug 11;17(8):1312. doi: 10.3390/ijms17081312. Int J Mol Sci. 2016. PMID: 27529213 Free PMC article. Review.
-
Kallistatin: double-edged role in angiogenesis, apoptosis and oxidative stress.Biol Chem. 2017 Nov 27;398(12):1309-1317. doi: 10.1515/hsz-2017-0180. Biol Chem. 2017. PMID: 28742513 Review.
Cited by
-
Kallistatin as a Potential Biomarker in Polycystic Ovary Syndrome: A Prospective Cohort Study.Diagnostics (Basel). 2024 Jul 18;14(14):1553. doi: 10.3390/diagnostics14141553. Diagnostics (Basel). 2024. PMID: 39061689 Free PMC article.
-
Systemic Markers of Lung Function and Forced Expiratory Volume in 1 Second Decline across Diverse Cohorts.Ann Am Thorac Soc. 2023 Aug;20(8):1124-1135. doi: 10.1513/AnnalsATS.202210-857OC. Ann Am Thorac Soc. 2023. PMID: 37351609 Free PMC article.
-
Differential epithelial and stromal protein profiles in cone and non-cone regions of keratoconus corneas.Sci Rep. 2019 Feb 27;9(1):2965. doi: 10.1038/s41598-019-39182-6. Sci Rep. 2019. PMID: 30814630 Free PMC article.
-
Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues.Pharmaceuticals (Basel). 2023 Mar 13;16(3):437. doi: 10.3390/ph16030437. Pharmaceuticals (Basel). 2023. PMID: 36986536 Free PMC article. Review.
-
Unveiling the levels and significance of different serpin family proteins in aqueous humor dynamics.BMC Ophthalmol. 2025 May 19;25(1):297. doi: 10.1186/s12886-025-04119-3. BMC Ophthalmol. 2025. PMID: 40389884 Free PMC article.
References
-
- Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, Wang LX, Lu HS, Chao L. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem. 1990;265:16394–16401. - PubMed
-
- Chai KX, Ma JX, Murray SR, Chao J, Chao L. Molecular cloning and analysis of the rat kallikrein-binding protein gene. J Biol Chem. 1991;266:16029–16036. - PubMed
-
- Chai KX, Chao J, Chao L. Molecular cloning and sequence analysis of the mouse kallikrein-binding protein gene. Biochim Biophys Acta. 1991;1129:127–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

