Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma
- PMID: 27432875
- PMCID: PMC5034737
- DOI: 10.1182/blood-2016-02-699850
Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma
Abstract
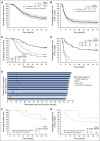
Presented here are the 5-year end-of-study results from the pivotal phase 2 trial of brentuximab vedotin in patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL) after failed hematopoietic autologous stem cell transplantation. At 5 years, the overall patient population (N = 102) had an estimated overall survival (OS) rate of 41% (95% confidence interval [CI]: 31-51) and progression-free survival (PFS) rate of 22% (95% CI: 13-31). Patients who achieved a complete response (CR) to brentuximab vedotin (N = 34) had estimated OS and PFS rates of 64% (95% CI: 48-80%) and 52% (95% CI: 34-69%), respectively. The median OS and PFS were not reached in CR patients, with 13 patients (38% of all CR patients) remaining in follow-up and in remission at study closure. Of the 13 patients, 4 received consolidative hematopoietic allogeneic stem cell transplant, and 9 (9% of all enrolled patients) remain in sustained CR without receiving any further anticancer therapy after treatment with brentuximab vedotin. Of the patients who experienced treatment-emergent peripheral neuropathy, 88% experienced either resolution (73%) or improvement (14%) in symptoms. These 5-year follow-up data demonstrate that a subset of patients with R/R HL who obtained CR with single-agent brentuximab vedotin achieved long-term disease control and may potentially be cured. The trial was registered at www.clinicaltrials.gov as #NCT00848926.
© 2016 by The American Society of Hematology.
Figures
Comment in
-
BV for HL: can the responses last?Blood. 2016 Sep 22;128(12):1540-1. doi: 10.1182/blood-2016-08-730374. Blood. 2016. PMID: 27658697 No abstract available.
References
-
- Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–10547. - PubMed
-
- Oflazoglu E, Stone IJ, Gordon KA, et al. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 2007;110(13):4370–4372. - PubMed
-
- Müller P, Martin K, Theurich S, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2(8):741–755. - PubMed
-
- Gardai SJ, Epp A, Law C-L. Brentuximab vedotin-mediated immunogenic cell death [abstract]. Cancer Res. 2015 75(15 Suppl). Abstract 2469.
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

