An Oral Load of [13C3]Glycerol and Blood NMR Analysis Detect Fatty Acid Esterification, Pentose Phosphate Pathway, and Glycerol Metabolism through the Tricarboxylic Acid Cycle in Human Liver
- PMID: 27432878
- PMCID: PMC5009274
- DOI: 10.1074/jbc.M116.742262
An Oral Load of [13C3]Glycerol and Blood NMR Analysis Detect Fatty Acid Esterification, Pentose Phosphate Pathway, and Glycerol Metabolism through the Tricarboxylic Acid Cycle in Human Liver
Abstract
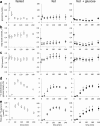
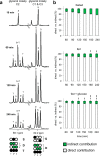
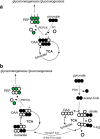
Drugs and other interventions for high impact hepatic diseases often target biochemical pathways such as gluconeogenesis, lipogenesis, or the metabolic response to oxidative stress. However, traditional liver function tests do not provide quantitative data about these pathways. In this study, we developed a simple method to evaluate these processes by NMR analysis of plasma metabolites. Healthy subjects ingested [U-(13)C3]glycerol, and blood was drawn at multiple times. Each subject completed three visits under differing nutritional states. High resolution (13)C NMR spectra of plasma triacylglycerols and glucose provided new insights into a number of hepatic processes including fatty acid esterification, the pentose phosphate pathway, and gluconeogenesis through the tricarboxylic acid cycle. Fasting stimulated pentose phosphate pathway activity and metabolism of [U-(13)C3]glycerol in the tricarboxylic acid cycle prior to gluconeogenesis or glyceroneogenesis. Fatty acid esterification was transient in the fasted state but continuous under fed conditions. We conclude that a simple NMR analysis of blood metabolites provides an important biomarker of pentose phosphate pathway activity, triacylglycerol synthesis, and flux through anaplerotic pathways in mitochondria of human liver.
Keywords: anaplerosis; biomarker; gluconeogenesis; glycerol; liver metabolism; mitochondria; pentose phosphate pathway (PPP); tricarboxylic acid cycle (TCA cycle) (Krebs cycle); triglyceride.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
A simple method to monitor hepatic gluconeogenesis and triglyceride synthesis following oral sugar tolerance test in obese adolescents.Am J Physiol Regul Integr Comp Physiol. 2019 Jul 1;317(1):R134-R142. doi: 10.1152/ajpregu.00047.2019. Epub 2019 May 1. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 31042400 Free PMC article.
-
Interaction between the pentose phosphate pathway and gluconeogenesis from glycerol in the liver.J Biol Chem. 2014 Nov 21;289(47):32593-603. doi: 10.1074/jbc.M114.577692. Epub 2014 Oct 6. J Biol Chem. 2014. PMID: 25288790 Free PMC article.
-
Effects of visceral adiposity on glycerol pathways in gluconeogenesis.Metabolism. 2017 Feb;67:80-89. doi: 10.1016/j.metabol.2016.11.008. Epub 2016 Nov 27. Metabolism. 2017. PMID: 28081781 Free PMC article.
-
A reverse KREBS cycle in photosynthesis: consensus at last.Photosynth Res. 1990;24:47-53. Photosynth Res. 1990. PMID: 11540925 Review.
-
Bonded cumomer analysis of tumor metabolism based on 13 C magnetic resonance spectroscopy.NMR Biomed. 2023 Apr;36(4):e4716. doi: 10.1002/nbm.4716. Epub 2022 Mar 17. NMR Biomed. 2023. PMID: 35196744 Review.
Cited by
-
Fatty liver disrupts glycerol metabolism in gluconeogenic and lipogenic pathways in humans.J Lipid Res. 2018 Sep;59(9):1685-1694. doi: 10.1194/jlr.M086405. Epub 2018 Jul 27. J Lipid Res. 2018. PMID: 30054343 Free PMC article.
-
Pentose phosphate pathway activity parallels lipogenesis but not antioxidant processes in rat liver.Am J Physiol Endocrinol Metab. 2018 Jun 1;314(6):E543-E551. doi: 10.1152/ajpendo.00342.2017. Epub 2018 Jan 9. Am J Physiol Endocrinol Metab. 2018. PMID: 29351478 Free PMC article.
-
A randomized clinical trial evaluating the effect of empagliflozin on triglycerides in obese adults: Role of visceral fat.Metabol Open. 2021 Dec 25;13:100161. doi: 10.1016/j.metop.2021.100161. eCollection 2022 Mar. Metabol Open. 2021. PMID: 35024596 Free PMC article.
-
tcaSIM: A Simulation Program for Optimal Design of 13C Tracer Experiments for Analysis of Metabolic Flux by NMR and Mass Spectroscopy.Curr Metabolomics. 2018;6(3):176-187. doi: 10.2174/2213235X07666181219115856. Curr Metabolomics. 2018. PMID: 31745452 Free PMC article.
-
A simple method to monitor hepatic gluconeogenesis and triglyceride synthesis following oral sugar tolerance test in obese adolescents.Am J Physiol Regul Integr Comp Physiol. 2019 Jul 1;317(1):R134-R142. doi: 10.1152/ajpregu.00047.2019. Epub 2019 May 1. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 31042400 Free PMC article.
References
-
- Neuschwander-Tetri B. A., Loomba R., Sanyal A. J., Lavine J. E., Van Natta M. L., Abdelmalek M. F., Chalasani N., Dasarathy S., Diehl A. M., Hameed B., Kowdley K. V., McCullough A., Terrault N., Clark J. M., Tonascia J., Brunt E. M., Kleiner D. E., Doo E, and NASH Clinical Research Network (2015) Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

