Selective Inhibition of Human Equilibrative and Concentrative Nucleoside Transporters by BCR-ABL Kinase Inhibitors: IDENTIFICATION OF KEY hENT1 AMINO ACID RESIDUES FOR INTERACTION WITH BCR-ABL KINASE INHIBITORS
- PMID: 27432881
- PMCID: PMC5009255
- DOI: 10.1074/jbc.M116.741074
Selective Inhibition of Human Equilibrative and Concentrative Nucleoside Transporters by BCR-ABL Kinase Inhibitors: IDENTIFICATION OF KEY hENT1 AMINO ACID RESIDUES FOR INTERACTION WITH BCR-ABL KINASE INHIBITORS
Abstract
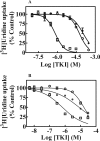
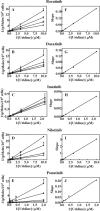
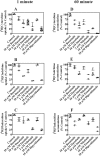
Human nucleoside transporters (hNTs) mediate cellular influx of anticancer nucleoside drugs, including cytarabine, cladribine, and fludarabine. BCR-ABL tyrosine kinase inhibitors (TKIs) imatinib and dasatinib inhibit fludarabine and cytarabine uptake. We assessed interactions of bosutinib, dasatinib, imatinib, nilotinib, and ponatinib with recombinant hNTs (hENT1, 2; hCNT1, -2, and -3) produced individually in yeast Saccharomyces cerevisiae Nilotinib inhibited hENT1-mediated uridine transport most potently (IC50 value, 0.7 μm) followed by ponatinib > bosutinib > dasatinib > imatinib. Imatinib inhibited hCNT2 with an IC50 value of 2.3 μm Ponatinib inhibited all five hNTs with the greatest effect seen for hENT1 (IC50 value, 9 μm). TKIs inhibited [(3)H]uridine uptake in a competitive manner. Studies in yeast with mutants at two amino acid residues of hENT1 (L442I, L442T, M33A, M33A/L442I) previously shown to be involved in uridine and dipyridamole binding, suggested that BCR-ABL TKIs interacted with Met(33) (TM1) and Leu(442) (TM11) residues of hENT1. In cultured human CEM lymphoblastoid cells, which possess a single hNT type (hENT1), accumulation of [(3)H]cytarabine, [(3)H]cladribine, or [(3)H]fludarabine was reduced by each of the five TKIs, and also caused a reduction in cell surface expression of hENT1 protein. In conclusion, BCR-ABL TKIs variously inhibit five different hNTs, cause a decrease in cell surface hENT1 expression, and decrease uridine accumulation when presented together with uridine or when given before uridine. In experiments with mutant hENT1, we showed for the first time interaction of Met(33) (involved in dipyridamole binding) with BCR-ABL inhibitors and reduced interaction with M33A mutant hENT1.
Keywords: Saccharomyces cerevisiae; drug resistance; drug transport; inhibition mechanism; membrane transport; nucleoside/nucleotide transport; receptor tyrosine kinase.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors.Am J Hematol. 2012 Nov;87(11):E125-8. doi: 10.1002/ajh.23338. Epub 2012 Oct 9. Am J Hematol. 2012. PMID: 23044928
-
Mutation of leucine-92 selectively reduces the apparent affinity of inosine, guanosine, NBMPR [S6-(4-nitrobenzyl)-mercaptopurine riboside] and dilazep for the human equilibrative nucleoside transporter, hENT1.Biochem J. 2004 May 15;380(Pt 1):131-7. doi: 10.1042/BJ20031880. Biochem J. 2004. PMID: 14759222 Free PMC article.
-
Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1).J Biol Chem. 2011 Sep 16;286(37):32552-62. doi: 10.1074/jbc.M111.236117. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795683 Free PMC article.
-
BCR-ABL inhibitors in chronic myeloid leukemia: process chemistry and biochemical profile.Curr Med Chem. 2011;18(19):2943-59. doi: 10.2174/092986711796150414. Curr Med Chem. 2011. PMID: 21651486 Review.
-
Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy.J Hematol Oncol. 2018 Jun 20;11(1):84. doi: 10.1186/s13045-018-0624-2. J Hematol Oncol. 2018. PMID: 29925402 Free PMC article. Review.
Cited by
-
A review of the Augustine blood group system.Int J Hematol. 2024 Jul;120(1):44-49. doi: 10.1007/s12185-024-03791-3. Epub 2024 May 20. Int J Hematol. 2024. PMID: 38767828 Review.
-
Re-Discovery of Pyrimidine Salvage as Target in Cancer Therapy.Cells. 2022 Feb 20;11(4):739. doi: 10.3390/cells11040739. Cells. 2022. PMID: 35203388 Free PMC article. Review.
-
Heterologous (Over) Expression of Human SoLute Carrier (SLC) in Yeast: A Well-Recognized Tool for Human Transporter Function/Structure Studies.Life (Basel). 2022 Aug 8;12(8):1206. doi: 10.3390/life12081206. Life (Basel). 2022. PMID: 36013385 Free PMC article. Review.
-
Modulating pyrimidine ribonucleotide levels for the treatment of cancer.Cancer Metab. 2020 Oct 4;8:12. doi: 10.1186/s40170-020-00218-5. eCollection 2020. Cancer Metab. 2020. PMID: 33020720 Free PMC article. Review.
-
Exploitation of dihydroorotate dehydrogenase (DHODH) and p53 activation as therapeutic targets: A case study in polypharmacology.J Biol Chem. 2020 Dec 25;295(52):17935-17949. doi: 10.1074/jbc.RA119.012056. Epub 2020 Sep 8. J Biol Chem. 2020. PMID: 32900849 Free PMC article.
References
-
- Ohanian M., Cortes J., Kantarjian H., and Jabbour E. (2012) Tyrosine kinase inhibitors in acute and chronic leukemias. Expert. Opin. Pharmacother. 13, 927–938 - PubMed
-
- Druker B. J., Sawyers C. L., Kantarjian H., Resta D. J., Reese S. F., Ford J. M., Capdeville R., and Talpaz M. (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 - PubMed
-
- Yang K., and Fu L. W. (2015) Mechanisms of resistance to BCR-ABL TKIs and the therapeutic strategies: a review. Crit. Rev. Oncol. Hematol. 93, 277–292 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

