Convergent Signaling Pathways Regulate Parathyroid Hormone and Fibroblast Growth Factor-23 Action on NPT2A-mediated Phosphate Transport
- PMID: 27432882
- PMCID: PMC5009241
- DOI: 10.1074/jbc.M116.744052
Convergent Signaling Pathways Regulate Parathyroid Hormone and Fibroblast Growth Factor-23 Action on NPT2A-mediated Phosphate Transport
Abstract
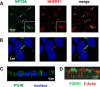
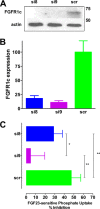
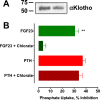
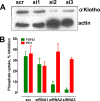
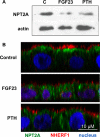
Parathyroid hormone (PTH) and FGF23 are the primary hormones regulating acute phosphate homeostasis. Human renal proximal tubule cells (RPTECs) were used to characterize the mechanism and signaling pathways of PTH and FGF23 on phosphate transport and the role of the PDZ protein NHERF1 in mediating PTH and FGF23 effects. RPTECs express the NPT2A phosphate transporter, αKlotho, FGFR1, FGFR3, FGFR4, and the PTH receptor. FGFR1 isoforms are formed from alternate splicing of exon 3 and of exon 8 or 9 in Ir-like loop 3. Exon 3 was absent, but mRNA containing both exons 8 and 9 is present in cytoplasm. Using an FGFR1c-specific antibody together with mass spectrometry analysis, we show that RPTECs express FGFR-β1C. The data are consistent with regulated FGFR1 splicing involving a novel cytoplasmic mechanism. PTH and FGF23 inhibited phosphate transport in a concentration-dependent manner. At maximally effective concentrations, PTH and FGF23 equivalently decreased phosphate uptake and were not additive, suggesting a shared mechanism of action. Protein kinase A or C blockade prevented PTH but not FGF23 actions. Conversely, inhibiting SGK1, blocking FGFR dimerization, or knocking down Klotho expression disrupted FGF23 actions but did not interfere with PTH effects. C-terminal FGF23(180-251) competitively and selectively blocked FGF23 action without disrupting PTH effects. However, both PTH and FGF23-sensitive phosphate transport were abolished by NHERF1 shRNA knockdown. Extended treatment with PTH or FGF23 down-regulated NPT2A without affecting NHERF1. We conclude that FGFR1c and PTHR signaling pathways converge on NHERF1 to inhibit PTH- and FGF23-sensitive phosphate transport and down-regulate NPT2A.
Keywords: G protein-coupled receptor (GPCR); NHERF1; NPT2A; PDZ Protein; alternative splicing; fibroblast growth factor receptor (FGFR); klotho; parathyroid hormone; transport.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
RGS14 regulates PTH- and FGF23-sensitive NPT2A-mediated renal phosphate uptake via binding to the NHERF1 scaffolding protein.J Biol Chem. 2022 May;298(5):101836. doi: 10.1016/j.jbc.2022.101836. Epub 2022 Mar 17. J Biol Chem. 2022. PMID: 35307350 Free PMC article.
-
Multisite NHERF1 phosphorylation controls GRK6A regulation of hormone-sensitive phosphate transport.J Biol Chem. 2021 Jan-Jun;296:100473. doi: 10.1016/j.jbc.2021.100473. Epub 2021 Feb 24. J Biol Chem. 2021. PMID: 33639163 Free PMC article.
-
Distinct and overlapping RGS14 and RGS12 actions regulate NPT2A-mediated phosphate transport.Biochem Biophys Res Commun. 2024 Nov 12;733:150700. doi: 10.1016/j.bbrc.2024.150700. Epub 2024 Sep 14. Biochem Biophys Res Commun. 2024. PMID: 39293332
-
Noncanonical Sequences Involving NHERF1 Interaction with NPT2A Govern Hormone-Regulated Phosphate Transport: Binding Outside the Box.Int J Mol Sci. 2021 Jan 22;22(3):1087. doi: 10.3390/ijms22031087. Int J Mol Sci. 2021. PMID: 33499384 Free PMC article. Review.
-
Pleiotropic Actions of FGF23.Toxicol Pathol. 2017 Oct;45(7):904-910. doi: 10.1177/0192623317737469. Epub 2017 Nov 2. Toxicol Pathol. 2017. PMID: 29096595 Free PMC article. Review.
Cited by
-
The Role of Fibroblast Growth Factor 23 in Inflammation and Anemia.Int J Mol Sci. 2019 Aug 27;20(17):4195. doi: 10.3390/ijms20174195. Int J Mol Sci. 2019. PMID: 31461904 Free PMC article. Review.
-
FGF23 Actions on Target Tissues-With and Without Klotho.Front Endocrinol (Lausanne). 2018 May 2;9:189. doi: 10.3389/fendo.2018.00189. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29770125 Free PMC article. Review.
-
Control of phosphate balance by the kidney and intestine.Clin Exp Nephrol. 2017 Mar;21(Suppl 1):21-26. doi: 10.1007/s10157-016-1359-4. Epub 2016 Nov 30. Clin Exp Nephrol. 2017. PMID: 27900568 Review.
-
The Complexities of Organ Crosstalk in Phosphate Homeostasis: Time to Put Phosphate Sensing Back in the Limelight.Int J Mol Sci. 2021 May 27;22(11):5701. doi: 10.3390/ijms22115701. Int J Mol Sci. 2021. PMID: 34071837 Free PMC article. Review.
-
RGS14 regulates PTH- and FGF23-sensitive NPT2A-mediated renal phosphate uptake via binding to the NHERF1 scaffolding protein.J Biol Chem. 2022 May;298(5):101836. doi: 10.1016/j.jbc.2022.101836. Epub 2022 Mar 17. J Biol Chem. 2022. PMID: 35307350 Free PMC article.
References
-
- Biber J., Malmström K., Reshkin S., and Murer H. (1990) Phosphate transport in established renal epithelial cell lines. Methods Enzymol. 191, 494–505 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

