Association Between Results of a Gene Expression Signature Assay and Recurrence-Free Interval in Patients With Stage II Colon Cancer in Cancer and Leukemia Group B 9581 (Alliance)
- PMID: 27432924
- PMCID: PMC5012711
- DOI: 10.1200/JCO.2015.65.4699
Association Between Results of a Gene Expression Signature Assay and Recurrence-Free Interval in Patients With Stage II Colon Cancer in Cancer and Leukemia Group B 9581 (Alliance)
Abstract
Purpose: Conventional staging methods are inadequate to identify patients with stage II colon cancer (CC) who are at high risk of recurrence after surgery with curative intent. ColDx is a gene expression, microarray-based assay shown to be independently prognostic for recurrence-free interval (RFI) and overall survival in CC. The objective of this study was to further validate ColDx using formalin-fixed, paraffin-embedded specimens collected as part of the Alliance phase III trial, C9581.
Patients and methods: C9581 evaluated edrecolomab versus observation in patients with stage II CC and reported no survival benefit. Under an initial case-cohort sampling design, a randomly selected subcohort (RS) comprised 514 patients from 901 eligible patients with available tissue. Forty-nine additional patients with recurrence events were included in the analysis. Final analysis comprised 393 patients: 360 RS (58 events) and 33 non-RS events. Risk status was determined for each patient by ColDx. The Self-Prentice method was used to test the association between the resulting ColDx risk score and RFI adjusting for standard prognostic variables.
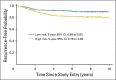
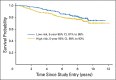
Results: Fifty-five percent of patients (216 of 393) were classified as high risk. After adjustment for prognostic variables that included mismatch repair (MMR) deficiency, ColDx high-risk patients exhibited significantly worse RFI (multivariable hazard ratio, 2.13; 95% CI, 1.3 to 3.5; P < .01). Age and MMR status were marginally significant. RFI at 5 years for patients classified as high risk was 82% (95% CI, 79% to 85%), compared with 91% (95% CI, 89% to 93%) for patients classified as low risk.
Conclusion: ColDx is associated with RFI in the C9581 subsample in the presence of other prognostic factors, including MMR deficiency. ColDx could be incorporated with the traditional clinical markers of risk to refine patient prognosis.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures


Comment in
-
Can Colon Cancer Recurrence and Metastases Be Determined After Surgical Resection Using a Gene Expression Signature?J Clin Oncol. 2017 Apr 20;35(12):1372-1373. doi: 10.1200/JCO.2016.71.1572. Epub 2017 Jan 23. J Clin Oncol. 2017. PMID: 28113016 No abstract available.
-
Reply to L. Casadaban et al.J Clin Oncol. 2017 Apr 20;35(12):1373-1374. doi: 10.1200/JCO.2016.71.2646. Epub 2017 Jan 23. J Clin Oncol. 2017. PMID: 28113023 No abstract available.
References
-
- Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer; 2010.
-
- Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. - PubMed
-
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology, Colon Cancer v2.2015. http://www.nccn.org. - PubMed
-
- Van Cutsem E, Oliveria J, ESMO Guidelines Working Group: Primary colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol 20:49-50, 2009 (suppl 4) - PubMed
-
- Vicuna B, Benson AB., III Adjuvant therapy for stage II colon cancer: Prognostic and predictive markers. J Natl Compr Canc Netw. 2007;5:927–936. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

