Changes in insulin and insulin signaling in Alzheimer's disease: cause or consequence?
- PMID: 27432942
- PMCID: PMC4986537
- DOI: 10.1084/jem.20160493
Changes in insulin and insulin signaling in Alzheimer's disease: cause or consequence?
Abstract
Individuals with type 2 diabetes have an increased risk for developing Alzheimer's disease (AD), although the causal relationship remains poorly understood. Alterations in insulin signaling (IS) are reported in the AD brain. Moreover, oligomers/fibrils of amyloid-β (Aβ) can lead to neuronal insulin resistance and intranasal insulin is being explored as a potential therapy for AD. Conversely, elevated insulin levels (ins) are found in AD patients and high insulin has been reported to increase Aβ levels and tau phosphorylation, which could exacerbate AD pathology. Herein, we explore whether changes in ins and IS are a cause or consequence of AD.
© 2016 Stanley et al.
Figures
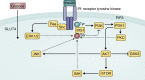

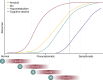
References
-
- Baker L.D., Cross D.J., Minoshima S., Belongia D., Watson G.S., and Craft S.. 2011. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68:51–57. 10.1001/archneurol.2010.225 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

