N-methylation of a bactericidal compound as a resistance mechanism in Mycobacterium tuberculosis
- PMID: 27432954
- PMCID: PMC4978242
- DOI: 10.1073/pnas.1606590113
N-methylation of a bactericidal compound as a resistance mechanism in Mycobacterium tuberculosis
Abstract
The rising incidence of antimicrobial resistance (AMR) makes it imperative to understand the underlying mechanisms. Mycobacterium tuberculosis (Mtb) is the single leading cause of death from a bacterial pathogen and estimated to be the leading cause of death from AMR. A pyrido-benzimidazole, 14, was reported to have potent bactericidal activity against Mtb. Here, we isolated multiple Mtb clones resistant to 14. Each had mutations in the putative DNA-binding and dimerization domains of rv2887, a gene encoding a transcriptional repressor of the MarR family. The mutations in Rv2887 led to markedly increased expression of rv0560c. We characterized Rv0560c as an S-adenosyl-L-methionine-dependent methyltransferase that N-methylates 14, abolishing its mycobactericidal activity. An Mtb strain lacking rv0560c became resistant to 14 by mutating decaprenylphosphoryl-β-d-ribose 2-oxidase (DprE1), an essential enzyme in arabinogalactan synthesis; 14 proved to be a nanomolar inhibitor of DprE1, and methylation of 14 by Rv0560c abrogated this activity. Thus, 14 joins a growing list of DprE1 inhibitors that are potently mycobactericidal. Bacterial methylation of an antibacterial agent, 14, catalyzed by Rv0560c of Mtb, is a previously unreported mechanism of AMR.
Keywords: antimicrobial resistance; arabinogalactan synthesis; methyltransferase; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

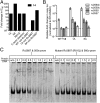
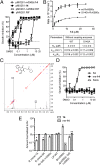

Comment in
-
Antibiotic Methylation: A New Mechanism of Antimicrobial Resistance.Trends Microbiol. 2016 Oct;24(10):771-772. doi: 10.1016/j.tim.2016.08.003. Epub 2016 Sep 1. Trends Microbiol. 2016. PMID: 27593675
References
-
- World Health Organization . Global Tuberculosis Report 2014. WHO Press; Geneva: 2014.
-
- World Health Organization . Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. WHO Press; Geneva: 2011. - PubMed
-
- Pietersen E, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: A cohort study. Lancet. 2014;383(9924):1230–1239. - PubMed
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10) Suppl 2:S231–S279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

