Superpriming of synaptic vesicles as a common basis for intersynapse variability and modulation of synaptic strength
- PMID: 27432975
- PMCID: PMC4978258
- DOI: 10.1073/pnas.1606383113
Superpriming of synaptic vesicles as a common basis for intersynapse variability and modulation of synaptic strength
Abstract
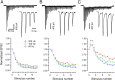
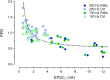
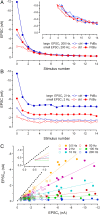
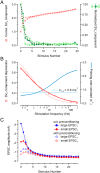
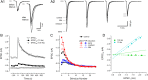
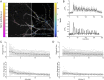
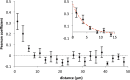
Glutamatergic synapses show large variations in strength and short-term plasticity (STP). We show here that synapses displaying an increased strength either after posttetanic potentiation (PTP) or through activation of the phospholipase-C-diacylglycerol pathway share characteristic properties with intrinsically strong synapses, such as (i) pronounced short-term depression (STD) during high-frequency stimulation; (ii) a conversion of that STD into a sequence of facilitation followed by STD after a few conditioning stimuli at low frequency; (iii) an equalizing effect of such conditioning stimulation, which reduces differences among synapses and abolishes potentiation; and (iv) a requirement of long periods of rest for reconstitution of the original STP pattern. These phenomena are quantitatively described by assuming that a small fraction of "superprimed" synaptic vesicles are in a state of elevated release probability (p ∼ 0.5). This fraction is variable in size among synapses (typically about 30%), but increases after application of phorbol ester or during PTP. The majority of vesicles, released during repetitive stimulation, have low release probability (p ∼ 0.1), are relatively uniform in number across synapses, and are rapidly recruited. In contrast, superprimed vesicles need several seconds to be regenerated. They mediate enhanced synaptic strength at the onset of burst-like activity, the impact of which is subject to modulation by slow modulatory transmitter systems.
Keywords: Munc13; calyx of Held; phorbol ester; posttetanic potentiation; short-term plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held.J Neurosci. 2005 May 25;25(21):5127-37. doi: 10.1523/JNEUROSCI.1295-05.2005. J Neurosci. 2005. PMID: 15917453 Free PMC article.
-
A Slow Short-Term Depression at Purkinje to Deep Cerebellar Nuclear Neuron Synapses Supports Gain-Control and Linear Encoding over Second-Long Time Windows.J Neurosci. 2020 Jul 29;40(31):5937-5953. doi: 10.1523/JNEUROSCI.2078-19.2020. Epub 2020 Jun 17. J Neurosci. 2020. PMID: 32554551 Free PMC article.
-
The reduced release probability of releasable vesicles during recovery from short-term synaptic depression.Neuron. 1999 Aug;23(4):821-32. doi: 10.1016/s0896-6273(01)80039-8. Neuron. 1999. PMID: 10482247
-
Estimation of quantal parameters at the calyx of Held synapse.Neurosci Res. 2002 Dec;44(4):343-56. doi: 10.1016/s0168-0102(02)00174-8. Neurosci Res. 2002. PMID: 12445623 Review.
-
Multiple vesicle recycling pathways in central synapses and their impact on neurotransmission.J Physiol. 2007 Dec 15;585(Pt 3):669-79. doi: 10.1113/jphysiol.2007.137745. Epub 2007 Aug 9. J Physiol. 2007. PMID: 17690145 Free PMC article. Review.
Cited by
-
Different priming states of synaptic vesicles underlie distinct release probabilities at hippocampal excitatory synapses.Neuron. 2022 Dec 21;110(24):4144-4161.e7. doi: 10.1016/j.neuron.2022.09.035. Epub 2022 Oct 18. Neuron. 2022. PMID: 36261033 Free PMC article.
-
Fully-primed slowly-recovering vesicles mediate presynaptic LTP at neocortical neurons.Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2305460120. doi: 10.1073/pnas.2305460120. Epub 2023 Oct 19. Proc Natl Acad Sci U S A. 2023. PMID: 37856547 Free PMC article.
-
Optical measurement of glutamate release robustly reports short-term plasticity at a fast central synapse.Front Mol Neurosci. 2024 Feb 28;17:1351280. doi: 10.3389/fnmol.2024.1351280. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38481471 Free PMC article.
-
The control of release probability at nerve terminals.Nat Rev Neurosci. 2019 Mar;20(3):177-186. doi: 10.1038/s41583-018-0111-3. Nat Rev Neurosci. 2019. PMID: 30647451 Review.
-
Doc2-mediated superpriming supports synaptic augmentation.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5605-E5613. doi: 10.1073/pnas.1802104115. Epub 2018 May 29. Proc Natl Acad Sci U S A. 2018. PMID: 29844163 Free PMC article.
References
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

