Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase
- PMID: 27432987
- PMCID: PMC4978270
- DOI: 10.1073/pnas.1604936113
Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase
Abstract
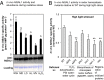
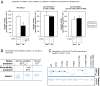
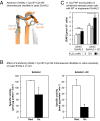
Intracellular signaling during oxidative stress is complex, with organelle-to-nucleus retrograde communication pathways ill-defined or incomplete. Here we identify the 3'-phosphoadenosine 5'-phosphate (PAP) phosphatase SAL1 as a previously unidentified and conserved oxidative stress sensor in plant chloroplasts. Arabidopsis thaliana SAL1 (AtSAL1) senses changes in photosynthetic redox poise, hydrogen peroxide, and superoxide concentrations in chloroplasts via redox regulatory mechanisms. AtSAL1 phosphatase activity is suppressed by dimerization, intramolecular disulfide formation, and glutathionylation, allowing accumulation of its substrate, PAP, a chloroplast stress retrograde signal that regulates expression of plastid redox associated nuclear genes (PRANGs). This redox regulation of SAL1 for activation of chloroplast signaling is conserved in the plant kingdom, and the plant protein has evolved enhanced redox sensitivity compared with its yeast ortholog. Our results indicate that in addition to sulfur metabolism, SAL1 orthologs have evolved secondary functions in oxidative stress sensing in the plant kingdom.
Keywords: chloroplast; drought stress; redox regulation; retrograde signaling; stress sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis.Plant Cell. 2011 Nov;23(11):3992-4012. doi: 10.1105/tpc.111.091033. Epub 2011 Nov 29. Plant Cell. 2011. PMID: 22128124 Free PMC article.
-
The PAP/SAL1 retrograde signaling pathway is involved in iron homeostasis.Plant Mol Biol. 2020 Feb;102(3):323-337. doi: 10.1007/s11103-019-00950-7. Epub 2020 Jan 3. Plant Mol Biol. 2020. PMID: 31900819
-
3'-Phosphoadenosine 5'-Phosphate Accumulation Delays the Circadian System.Plant Physiol. 2018 Apr;176(4):3120-3135. doi: 10.1104/pp.17.01611. Epub 2018 Feb 27. Plant Physiol. 2018. PMID: 29487119 Free PMC article.
-
Secondary sulfur metabolism in cellular signalling and oxidative stress responses.J Exp Bot. 2019 Aug 19;70(16):4237-4250. doi: 10.1093/jxb/erz119. J Exp Bot. 2019. PMID: 30868163 Review.
-
Plastidial retrograde modulation of light and hormonal signaling: an odyssey.New Phytol. 2021 May;230(3):931-937. doi: 10.1111/nph.17192. Epub 2021 Feb 4. New Phytol. 2021. PMID: 33452833 Review.
Cited by
-
Hepatitis C Virus RNA-Dependent RNA Polymerase Is Regulated by Cysteine S-Glutathionylation.Oxid Med Cell Longev. 2019 Sep 3;2019:3196140. doi: 10.1155/2019/3196140. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31687077 Free PMC article.
-
SAL1-PAP retrograde signalling extends circadian period by reproducing the loss of exoribonuclease (XRN) activity.Plant Signal Behav. 2018;13(8):e1500066. doi: 10.1080/15592324.2018.1500066. Epub 2018 Aug 6. Plant Signal Behav. 2018. PMID: 30081763 Free PMC article.
-
Reactive oxygen species signalling in plant stress responses.Nat Rev Mol Cell Biol. 2022 Oct;23(10):663-679. doi: 10.1038/s41580-022-00499-2. Epub 2022 Jun 27. Nat Rev Mol Cell Biol. 2022. PMID: 35760900 Review.
-
Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment.Plants (Basel). 2025 Aug 8;14(16):2464. doi: 10.3390/plants14162464. Plants (Basel). 2025. PMID: 40872087 Free PMC article. Review.
-
A mutation in Arabidopsis SAL1 alters its in vitro activity against IP3 and delays developmental leaf senescence in association with lower ROS levels.Plant Mol Biol. 2022 Apr;108(6):549-563. doi: 10.1007/s11103-022-01245-0. Epub 2022 Feb 5. Plant Mol Biol. 2022. PMID: 35122174
References
-
- Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

