Effects of Chinese Propolis in Protecting Bovine Mammary Epithelial Cells against Mastitis Pathogens-Induced Cell Damage
- PMID: 27433029
- PMCID: PMC4940570
- DOI: 10.1155/2016/8028291
Effects of Chinese Propolis in Protecting Bovine Mammary Epithelial Cells against Mastitis Pathogens-Induced Cell Damage
Abstract
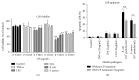
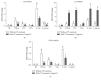
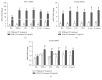
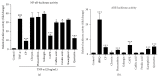
Chinese propolis (CP), an important hive product, can alleviate inflammatory responses. However, little is known regarding the potential of propolis treatment for mastitis control. To investigate the anti-inflammatory effects of CP on bovine mammary epithelial cells (MAC-T), we used a range of pathogens to induce cellular inflammatory damage. Cell viability was determined and expressions of inflammatory/antioxidant genes were measured. Using a cell-based reporter assay system, we evaluated CP and its primary constituents on the NF-κB and Nrf2-ARE transcription activation. MAC-T cells treated with bacterial endotoxin (lipopolysaccharide, LPS), heat-inactivated Escherichia coli, and Staphylococcus aureus exhibited significant decreases in cell viability while TNF-α and lipoteichoic acid (LTA) did not. Pretreatment with CP prevented losses in cell viability associated with the addition of killed bacteria or bacterial endotoxins. There were also corresponding decreases in expressions of proinflammatory IL-6 and TNF-α mRNA. Compared with the mastitis challenged cells, enhanced expressions of antioxidant genes HO-1, Txnrd-1, and GCLM were observed in CP-treated cells. CP and its polyphenolic active components (primarily caffeic acid phenethyl ester and quercetin) had strong inhibitive effects against NF-κB activation and increased the transcriptional activity of Nrf2-ARE. These findings suggest that propolis may be valuable in the control of bovine mastitis.
Figures

Similar articles
-
Bee Venom Decreases LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells.J Microbiol Biotechnol. 2017 Oct 28;27(10):1827-1836. doi: 10.4014/jmb.1706.06003. J Microbiol Biotechnol. 2017. PMID: 28813781
-
Prolactin-induced activation of nuclear factor kappaB in bovine mammary epithelial cells: role in chronic mastitis.J Dairy Sci. 2007 Jan;90(1):155-64. doi: 10.3168/jds.S0022-0302(07)72617-6. J Dairy Sci. 2007. PMID: 17183084
-
Bovine recombinant lipopolysaccharide binding protein (BRLBP) regulated apoptosis and inflammation response in lipopolysaccharide-challenged bovine mammary epithelial cells (BMEC).Mol Immunol. 2015 Jun;65(2):205-14. doi: 10.1016/j.molimm.2015.01.026. Epub 2015 Feb 18. Mol Immunol. 2015. PMID: 25700343
-
Transcriptome sequencing analysis of bovine mammary epithelial cells induced by lipopolysaccharide.Anim Biotechnol. 2024 Nov;35(1):2290527. doi: 10.1080/10495398.2023.2290527. Epub 2023 Dec 23. Anim Biotechnol. 2024. PMID: 38141161 Review.
-
Overview of Research Development on the Role of NF-κB Signaling in Mastitis.Animals (Basel). 2020 Sep 10;10(9):1625. doi: 10.3390/ani10091625. Animals (Basel). 2020. PMID: 32927884 Free PMC article. Review.
Cited by
-
Antibiotic-Antiapoptotic Dual Function of Clinacanthus nutans (Burm. f.) Lindau Leaf Extracts against Bovine Mastitis.Antibiotics (Basel). 2020 Jul 21;9(7):429. doi: 10.3390/antibiotics9070429. Antibiotics (Basel). 2020. PMID: 32708141 Free PMC article.
-
Transcriptome analysis identifies the NR4A subfamily involved in the alleviating effect of folic acid on mastitis induced by high concentration of Staphylococcus aureus lipoteichoic acid.BMC Genomics. 2024 Nov 7;25(1):1051. doi: 10.1186/s12864-024-10895-x. BMC Genomics. 2024. PMID: 39506684 Free PMC article.
-
Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments - A review.Asian-Australas J Anim Sci. 2020 Nov;33(11):1699-1713. doi: 10.5713/ajas.20.0156. Epub 2020 May 12. Asian-Australas J Anim Sci. 2020. PMID: 32777908 Free PMC article.
-
Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being.Pharmaceuticals (Basel). 2024 Aug 9;17(8):1050. doi: 10.3390/ph17081050. Pharmaceuticals (Basel). 2024. PMID: 39204155 Free PMC article. Review.
-
Targeting Nrf2/KEAP1 signaling pathway using bioactive compounds to combat mastitis.Front Immunol. 2025 Feb 7;16:1425901. doi: 10.3389/fimmu.2025.1425901. eCollection 2025. Front Immunol. 2025. PMID: 39991157 Free PMC article. Review.
References
-
- Kim K. W., Im J., Jeon J. H., Lee H.-G., Yun C.-H., Han S. H. Staphylococcus aureus induces IL-1β expression through the activation of MAP kinases and AP-1, CRE and NF-κB transcription factors in the bovine mammary gland epithelial cells. Comparative Immunology, Microbiology and Infectious Diseases. 2011;34(4):347–354. doi: 10.1016/j.cimid.2011.04.004. - DOI - PubMed
-
- Günther J., Esch K., Poschadel N., et al. Comparative kinetics of Escherichia coli- and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infection and Immunity. 2011;79(2):695–707. doi: 10.1128/iai.01071-10. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

