A Survey of Strategies to Modulate the Bone Morphogenetic Protein Signaling Pathway: Current and Future Perspectives
- PMID: 27433166
- PMCID: PMC4940573
- DOI: 10.1155/2016/7290686
A Survey of Strategies to Modulate the Bone Morphogenetic Protein Signaling Pathway: Current and Future Perspectives
Abstract
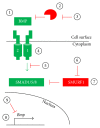
Bone morphogenetic proteins (BMPs) constitute the largest subdivision of the TGF-β family of ligands and are unequivocally involved in regulating stem cell behavior. Appropriate regulation of canonical BMP signaling is critical for the development and homeostasis of numerous human organ systems, as aberrations in the BMP pathway or its regulation are increasingly associated with diverse human pathologies. In this review, we provide a wide-perspective on strategies that increase or decrease BMP signaling. We briefly outline the current FDA-approved approaches, highlight emerging next-generation technologies, and postulate prospective avenues for future investigation. We also detail how activating other pathways may indirectly modulate BMP signaling, with a particular emphasis on the relationship between the BMP and Activin/TGF-β pathways.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

