Targeted therapies and immunotherapy in non-small-cell lung cancer
- PMID: 27433281
- PMCID: PMC4929979
- DOI: 10.3332/ecancer.2016.648
Targeted therapies and immunotherapy in non-small-cell lung cancer
Abstract
Non-small-cell lung cancer is still considered a difficult disease to manage because of its aggressiveness and resistance to common therapies. Chemotherapy remains the gold standard in nearly 80% of lung cancers, but clinical outcomes are discouraging, and the impact on median overall survival (OS) barely reaches 12 months. At the end of the last century, the discovery of oncogene-driven tumours completely changed the therapeutic landscape in lung cancers, harbouring specific gene mutations/translocations. Epidermal growth factors receptor (EGFR) common mutations first and anaplastic lymphoma kinase (ALK) translocations later led new insights in lung cancer biology knowledge. The use of specific tyrosine kinases inhibitors overturned the biological behaviour of EGFR mutation positive tumours and became a preclinical model to understand the heterogeneity of lung cancers and the mechanisms of drug resistance. In this review, we summarise the employment of targeted agents against the most representative biomolecular alterations and provide some criticisms of the therapeutic strategies.
Keywords: immunotherapy; non-small cell lung cancer; oncogene drivers; targeted agents.
Figures
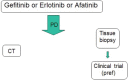
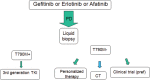
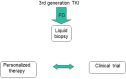
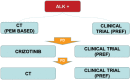
References
-
- Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
