Benzolamide improves oxygenation and reduces acute mountain sickness during a high-altitude trek and has fewer side effects than acetazolamide at sea level
- PMID: 27433337
- PMCID: PMC4876137
- DOI: 10.1002/prp2.203
Benzolamide improves oxygenation and reduces acute mountain sickness during a high-altitude trek and has fewer side effects than acetazolamide at sea level
Abstract
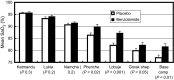
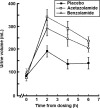
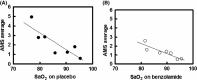
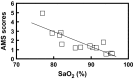
Acetazolamide is the standard carbonic anhydrase (CA) inhibitor used for acute mountain sickness (AMS), however some of its undesirable effects are related to intracellular penetrance into many tissues, including across the blood-brain barrier. Benzolamide is a much more hydrophilic inhibitor, which nonetheless retains a strong renal action to engender a metabolic acidosis and ventilatory stimulus that improves oxygenation at high altitude and reduces AMS. We tested the effectiveness of benzolamide versus placebo in a first field study of the drug as prophylaxis for AMS during an ascent to the Everest Base Camp (5340 m). In two other studies performed at sea level to test side effect differences between acetazolamide and benzolamide, we assessed physiological actions and psychomotor side effects of two doses of acetazolamide (250 and 1000 mg) in one group of healthy subjects and in another group compared acetazolamide (500 mg), benzolamide (200 mg) and lorazepam (2 mg) as an active comparator for central nervous system (CNS) effects. At high altitude, benzolamide-treated subjects maintained better arterial oxygenation at all altitudes (3-6% higher at all altitudes above 4200 m) than placebo-treated subjects and reduced AMS severity by roughly 50%. We found benzolamide had fewer side effects, some of which are symptoms of AMS, than any of the acetazolamide doses in Studies 1 and 2, but equal physiological effects on renal function. The psychomotor side effects of acetazolamide were dose dependent. We conclude that benzolamide is very effective for AMS prophylaxis. With its lesser CNS effects, benzolamide may be superior to acetazolamide, in part, because some of the side effects of acetazolamide may contribute to and be mistaken for AMS.
Keywords: Acetazolamide; acute mountain sickness; benzolamide; carbonic anhydrase inhibitor; high altitude; lorazepam; oxygen saturation; side effects.
Figures

References
-
- Bärtsch P, Swenson ER (2013). Clinical practice: acute high altitude illnesses. N Engl J Med 368: 2294–3027. - PubMed
-
- Basnyat B, Gertsch JH, Johnson EW, Castro‐Marin F, Inoue Y, Yeh C (2003). Efficacy of low‐dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double‐blind, randomized, placebo‐controlled trial. High Alt Med Biol 4: 45–52. - PubMed
-
- Basnyat B, Gertsch JH, Holck PS, Johnson W, Luks AM, Donham BP, et al. (2006). Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol 7: 17–27. - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. (1996). Improving the quality of reporting of randomized controlled trials: the CONSORT statement. J Am Med Assoc 276: 637–639. - PubMed
-
- Birmingham Medical Research Expeditionary Society Acute Mountain Sickness Study Group . 1981. Acetazolamide in control of acute mountain sickness. Lancet i:180–183.
LinkOut - more resources
Full Text Sources
Other Literature Sources

