Patterns of progression, treatment of progressive disease and post-progression survival in the New EPOC study
- PMID: 27434036
- PMCID: PMC4985352
- DOI: 10.1038/bjc.2016.208
Patterns of progression, treatment of progressive disease and post-progression survival in the New EPOC study
Abstract
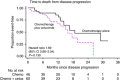
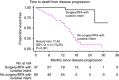
Background: The addition of cetuximab (CTX) to perioperative chemotherapy (CT) for operable colorectal liver metastases resulted in a shorter progression-free survival. Details of disease progression are described to further inform the primary study outcome.
Methods: A total of 257 KRAS wild-type patients were randomised to CT alone or CT with CTX. Data regarding sites and treatment of progressive disease were obtained for the 109 (CT n=48, CT and CTX n=61) patients with progressive disease at the cut-off date for analysis of November 2012.
Results: The liver was the most frequent site of progression (CT 67% (32/48); CT and CTX 66% (40/61)). A higher proportion of patients in the CT and group had multiple sites of progressive disease (CT 8%, 4/48; CT and CTX 23%, 14/61 P=0.04). Further treatment for progressive disease is known for 84 patients of whom 69 received further CT, most frequently irinotecan based. Twenty-two patients, 11 in each arm, received CTX as a further line agent.
Conclusions: Both the distribution of progressive disease and further treatment are as expected for such a cohort. The pattern of disease progression seen is consistent with failure of systemic micrometastatic disease control rather than failure of local disease control following liver surgery.
Conflict of interest statement
SF declared a consulting or advisory role to Merck. JV declared honoraria and travel/accommodation expenses from Merck. DO declared honoraria from Abbott and travel/accommodation expenses from Novartis and Angiodynamics. CR declared travel/accommodation expenses from Roche, Merck and Amgen. TI declared receiving honoraria from Roche and Celgene, a consulting or advisory role to Roche and Servier, and travel/accommodation expenses from Amgen and Bayer. OJG declared honoraria from Johnson and Johnson (Ethicon). DC declared receiving honoraria/research funding from Amgen, AstraZeneca, Bayer, Celgene, Medimmune, Merrimack, Merck Serono, and Sanofi. TM declared honoraria from Vertex, a consulting or advisory Role to Sanofi and research funding from AstraZeneca (Inst) and Merck Serono (Inst). JB declared receiving honoraria from Merck and Roche, attending a speakers' bureau for Merck Serono and receiving travel/accommodation expenses from Merck. The remaining authors declare no conflict of interest.
Figures
References
-
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer I (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23): 2343–2351. - PubMed
-
- de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250(3): 440–448. - PubMed
-
- Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, Brienza S, Alafaci E, Bertheault-Cvitkovic F, Jasmin C, Reynes M, Bismuth H, Misset JL, Levi F (1999) Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 10(6): 663–669. - PubMed
-
- Giessen C, Laubender RP, Ankerst DP, Stintzing S, Modest DP, Mansmann U, Heinemann V (2013) Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: literature-based analysis from 50 randomized first-line trials. Clin Cancer Res 19(1): 225–235. - PubMed
-
- Govaert KM, van Kessel CS, Steller EJ, Emmink BL, Molenaar IQ, Kranenburg O, van Hillegersberg R, Borel Rinkes IH (2014) Recurrence location after resection of colorectal liver metastases influences prognosis. J Gastrointest Surg 18(5): 952–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous