Hemolysis after Oral Artemisinin Combination Therapy for Uncomplicated Plasmodium falciparum Malaria
- PMID: 27434054
- PMCID: PMC4982175
- DOI: 10.3201/eid2208.151905
Hemolysis after Oral Artemisinin Combination Therapy for Uncomplicated Plasmodium falciparum Malaria
Abstract
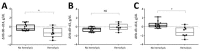
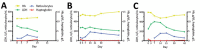
Episodes of delayed hemolysis 2-6 weeks after treatment of severe malaria with intravenous artesunate have been described. We performed a prospective observational study of patients with uncomplicated malaria to investigate whether posttreatment hemolysis also occurs after oral artemisinin-based combination therapy. Eight of 20 patients with uncomplicated malaria who were given oral artemisinin-based combination therapy met the definition of posttreatment hemolysis (low haptoglobin level and increased lactate dehydrogenase level on day 14). Five patients had hemolysis persisting for 1 month. Patients with posttreatment hemolysis had a median decrease in hemoglobin level of 1.3 g/dL (interquartile range 0.3-2.0 g/dL) in the posttreatment period, and patients without posttreatment hemolysis had a median increase of 0.3 g/dL (IQR -0.1 to 0.7 g/dL; p = 0.002). These findings indicate a need for increased vigilance for hemolytic events in malaria patients, particularly those with predisposing factors for anemia.
Keywords: Plasmodium falciparum; anemia; artemisinin; clinical study; haptoglobin; hemoglobin; hemolysis; lactate dehydrogenase; malaria; oral artemisinin combination therapy; parasites; uncomplicated malaria.
Figures

References
-
- World Health Organization. Guidelines for the treatment of malaria. 3rd ed. [cited 2016 Apr 15]. http://www.who.int/malaria/publications/atoz/9789241549127/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

