Hepatitis B Virus Middle Protein Enhances IL-6 Production via p38 MAPK/NF-κB Pathways in an ER Stress-Dependent Manner
- PMID: 27434097
- PMCID: PMC4951109
- DOI: 10.1371/journal.pone.0159089
Hepatitis B Virus Middle Protein Enhances IL-6 Production via p38 MAPK/NF-κB Pathways in an ER Stress-Dependent Manner
Abstract
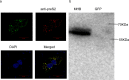
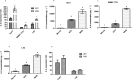
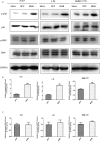
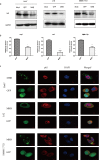
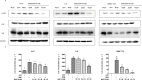
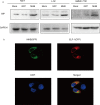
During hepatitis B virus (HBV) infection, three viral envelope proteins of HBV are overexpressed in the endoplasmic reticulum (ER). The large S protein (LHBs) and truncated middle S protein (MHBst) have been documented to play roles in regulating host gene expression and contribute to hepatic disease development. As a predominant protein at the ultrastructural level in biopsy samples taken from viremic patients, the role of the middle S protein (MHBs) remains to be understood despite its high immunogenicity. When we transfected hepatocytes with an enhanced green fluorescent protein (EGFP)-tagged MHBs expressing plasmid, the results showed that expression of MHBs cause an upregulation of IL-6 at the message RNA and protein levels through activating the p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor-kappa B (NF-κB) pathways. The use of specific inhibitors of the signaling pathways can diminish this upregulation. The use of BAPTA-AM attenuated the stimulation caused by MHBs. We further found that MHBs accumulated in the endoplasmic reticulum and increased the amount of glucose regulated protein 78 (GRP78/BiP). Our results provide a possibility that MHBs could be involved in liver disease progression.
Conflict of interest statement
Figures
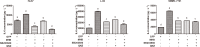
Similar articles
-
Exogenous hepatitis B virus envelope proteins induce endoplasmic reticulum stress: involvement of cannabinoid axis in liver cancer cells.Oncotarget. 2016 Apr 12;7(15):20312-23. doi: 10.18632/oncotarget.7950. Oncotarget. 2016. PMID: 26967385 Free PMC article.
-
Apolipoprotein H drives hepatitis B surface antigen retention and endoplasmic reticulum stress during hepatitis B virus infection.Int J Biochem Cell Biol. 2021 Feb;131:105906. doi: 10.1016/j.biocel.2020.105906. Epub 2020 Dec 26. Int J Biochem Cell Biol. 2021. PMID: 33370716
-
Possible Involvement of Multidrug-Resistant Hepatitis B Virus sW172* Truncation Variant in the ER Stress Signaling Pathway during Hepatocarcinogenesis.Jpn J Infect Dis. 2016 Jul 22;69(4):306-13. doi: 10.7883/yoken.JJID.2015.359. Epub 2015 Nov 13. Jpn J Infect Dis. 2016. PMID: 26567840
-
Hepatitis B Virus Core Antigen Stimulates IL-6 Expression via p38, ERK and NF-κB Pathways in Hepatocytes.Cell Physiol Biochem. 2017;41(1):91-100. doi: 10.1159/000455954. Epub 2017 Jan 17. Cell Physiol Biochem. 2017. PMID: 28214858
-
Interplay Between Non-Canonical NF-κB Signaling and Hepatitis B Virus Infection.Front Immunol. 2021 Sep 29;12:730684. doi: 10.3389/fimmu.2021.730684. eCollection 2021. Front Immunol. 2021. PMID: 34659217 Free PMC article. Review.
Cited by
-
Phosphoproteomics Unravel HBV Triggered Rewiring of Host Phosphosignaling Events.Int J Mol Sci. 2022 May 4;23(9):5127. doi: 10.3390/ijms23095127. Int J Mol Sci. 2022. PMID: 35563518 Free PMC article.
-
Regulation of Hepatitis B Virus Replication by Modulating Endoplasmic Reticulum Stress (ER-Stress).Int J Microbiol. 2024 Aug 30;2024:9117453. doi: 10.1155/2024/9117453. eCollection 2024. Int J Microbiol. 2024. PMID: 39246409 Free PMC article.
-
Increased BST-2 expression by HBV infection promotes HBV-associated HCC tumorigenesis.J Gastrointest Oncol. 2021 Apr;12(2):694-710. doi: 10.21037/jgo-20-356. J Gastrointest Oncol. 2021. PMID: 34012659 Free PMC article.
-
Mapping out p38MAPK.Am J Reprod Immunol. 2017 May;77(5):10.1111/aji.12652. doi: 10.1111/aji.12652. Epub 2017 Feb 13. Am J Reprod Immunol. 2017. PMID: 28194826 Free PMC article. Review.
-
Interleukin-35 Level Is Elevated in Patients with Chronic Hepatitis B Virus Infection.Int J Med Sci. 2018 Jan 1;15(2):188-194. doi: 10.7150/ijms.21957. eCollection 2018. Int J Med Sci. 2018. PMID: 29333103 Free PMC article.
References
-
- Dienes HP, Gerlich WH, Worsdorfer M, Gerken G, Bianchi L, Hess G, et al. (1990) Hepatic expression patterns of the large and middle hepatitis-B virus surface-proteins in viremic and nonviremic chronic hepatitis-B. Gastroenterology 98: 1017–1023. - PubMed
-
- Sengupta S, Rehman S, Durgapal H, Acharya SK, Panda SK (2007) Role of surface promoter mutations in hepatitis B surface antigen production and secretion in occult hepatitis B virus infection. Journal of medical virology 79: 220–228. - PubMed
-
- Pollicino T, Cacciola I, Saffioti F, Raimondo G (2014) Hepatitis B virus PreS/S gene variants: Pathobiology and clinical implications. Journal of hepatology. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

