Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells
- PMID: 27434269
- PMCID: PMC5072776
- DOI: 10.22203/ecm.v032a08
Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells
Abstract
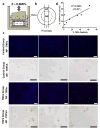
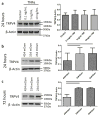
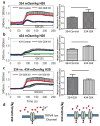
The mechanical behaviour and cellular metabolism of intervertebral discs (IVDs) and articular cartilage are strongly influenced by their proteoglycan content and associated osmotic properties. This osmotic environment is a biophysical signal that changes with disease and may contribute to the elevated matrix breakdown and altered biologic response to loading observed in IVD degeneration and osteoarthritis. This study tested the hypothesis that changes in osmo-sensation by the transient receptor potential vallinoid-4 (TRPV4) ion channel occur with disease and contribute to the inflammatory environment found during degeneration. Immunohistochemistry on bovine IVDs from an inflammatory organ culture model were used to investigate if TRPV4 is expressed in the IVD and how expression changes with degeneration. Western blot, live-cell calcium imaging, and qRT-PCR were used to investigate whether osmolarity changes or tumour necrosis factor α (TNFα) regulate TRPV4 expression, and how altered TRPV4 expression influences calcium signalling and pro-inflammatory cytokine expression. TRPV4 expression correlated with TNFα expression, and was increased when cultured in reduced medium osmolarity and unaltered with TNFα-stimulation. Increased TRPV4 expression increased the calcium flux following TRPV4 activation and increased interleukin-1β (IL-1β) and IL-6 gene expression in IVD cells. TRPV4 expression was qualitatively elevated in regions of aggrecan depletion in degenerated human IVDs. Collectively, results suggest that reduced tissue osmolarity, likely following proteoglycan degradation, can increase TRPV4 signalling and enhance pro-inflammatory cytokine production, suggesting changes in TRPV4 mediated osmo-sensation may contribute to the progressive matrix breakdown in disease.
Figures



Similar articles
-
Expression and Activity of TRPA1 and TRPV1 in the Intervertebral Disc: Association with Inflammation and Matrix Remodeling.Int J Mol Sci. 2019 Apr 10;20(7):1767. doi: 10.3390/ijms20071767. Int J Mol Sci. 2019. PMID: 30974795 Free PMC article.
-
Modulation of TRPV4 protects against degeneration induced by sustained loading and promotes matrix synthesis in the intervertebral disc.FASEB J. 2023 Feb;37(2):e22714. doi: 10.1096/fj.202201388R. FASEB J. 2023. PMID: 36583692 Free PMC article.
-
TNFα transport induced by dynamic loading alters biomechanics of intact intervertebral discs.PLoS One. 2015 Mar 3;10(3):e0118358. doi: 10.1371/journal.pone.0118358. eCollection 2015. PLoS One. 2015. PMID: 25734788 Free PMC article.
-
Osmosensing, osmosignalling and inflammation: how intervertebral disc cells respond to altered osmolarity.Eur Cell Mater. 2018 Nov 19;36:231-250. doi: 10.22203/eCM.v036a17. Eur Cell Mater. 2018. PMID: 30452080 Review.
-
Syndecan-4 in intervertebral disc and cartilage: Saint or synner?Matrix Biol. 2016 May-Jul;52-54:355-362. doi: 10.1016/j.matbio.2016.01.005. Epub 2016 Jan 18. Matrix Biol. 2016. PMID: 26796346 Free PMC article. Review.
Cited by
-
Hypo-Osmotic Loading Induces Expression of IL-6 in Nucleus Pulposus Cells of the Intervertebral Disc Independent of TRPV4 and TRPM7.Front Pharmacol. 2020 Jul 1;11:952. doi: 10.3389/fphar.2020.00952. eCollection 2020. Front Pharmacol. 2020. PMID: 32714187 Free PMC article.
-
Inhibition of Transient Receptor Potential Vanilloid 4 (TRPV4) Mitigates Seizures.Neurotherapeutics. 2022 Mar;19(2):660-681. doi: 10.1007/s13311-022-01198-8. Epub 2022 Feb 18. Neurotherapeutics. 2022. PMID: 35182379 Free PMC article.
-
Mechanotransduction and cell biomechanics of the intervertebral disc.JOR Spine. 2018 Sep;1(3):e1026. doi: 10.1002/jsp2.1026. Epub 2018 Jul 3. JOR Spine. 2018. PMID: 30569032 Free PMC article.
-
The role of TRPV4 in programmed cell deaths.Mol Biol Rep. 2024 Feb 1;51(1):248. doi: 10.1007/s11033-023-09199-2. Mol Biol Rep. 2024. PMID: 38300413 Review.
-
Transient receptor potential vanilloid 4 blockage attenuates pyroptosis in hippocampus of mice following pilocarpine‑induced status epilepticus.Acta Neuropathol Commun. 2025 Apr 10;13(1):73. doi: 10.1186/s40478-025-01990-5. Acta Neuropathol Commun. 2025. PMID: 40205503 Free PMC article.
References
-
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L158–172. - PMC - PubMed
-
- Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005;118:2435–2440. - PubMed
-
- Chen J, Baer AE, Paik PY, Yan W, Setton LA. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun. 2002;293:932–938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources