Coadministration of a Plasmid Encoding HIV-1 Gag Enhances the Efficacy of Cancer DNA Vaccines
- PMID: 27434590
- PMCID: PMC5113100
- DOI: 10.1038/mt.2016.122
Coadministration of a Plasmid Encoding HIV-1 Gag Enhances the Efficacy of Cancer DNA Vaccines
Abstract
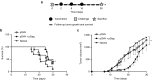
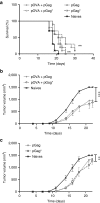
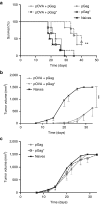
DNA vaccination holds great promise for the prevention and treatment of cancer and infectious diseases. However, the clinical ability of DNA vaccines is still controversial due to the limited immune response initially observed in humans. We hypothesized that electroporation of a plasmid encoding the HIV-1 Gag viral capsid protein would enhance cancer DNA vaccine potency. DNA electroporation used to deliver plasmids in vivo, induced type I interferons, thereby supporting the activation of innate immunity. The coadministration of ovalbumin (OVA) and HIV-1 Gag encoding plasmids modulated the adaptive immune response. This strategy favored antigen-specific Th1 immunity, delayed B16F10-OVA tumor growth and improved mouse survival in both prophylactic and therapeutic vaccination approaches. Similarly, a prophylactic DNA immunization against the melanoma-associated antigen gp100 was enhanced by the codelivery of the HIV-1 Gag plasmid. The adjuvant effect was not driven by the formation of HIV-1 Gag virus-like particles. This work highlights the ability of both electroporation and the HIV-1 Gag plasmid to stimulate innate immunity for enhancing cancer DNA vaccine immunogenicity and demonstrates interesting tracks for the design of new translational genetic adjuvants to overcome the current limitations of DNA vaccines in humans.
Figures

Similar articles
-
Improvement in T helper 1-related immune responses in BALB/c mice immunized with an HIV-1 gag plasmid combined with a chimeric plasmid encoding interleukin-18 and flagellin.Microbiol Immunol. 2015 Aug;59(8):483-94. doi: 10.1111/1348-0421.12274. Microbiol Immunol. 2015. PMID: 26094825
-
In vivo plasmid electroporation induces tumor antigen-specific CD8+ T-cell responses and delays tumor growth in a syngeneic mouse melanoma model.Cancer Res. 2002 Oct 1;62(19):5489-94. Cancer Res. 2002. PMID: 12359758
-
The improved antibody response against HIV-1 after a vaccination based on intrastructural help is complemented by functional CD8+ T cell responses.Vaccine. 2016 Apr 4;34(15):1744-51. doi: 10.1016/j.vaccine.2016.02.059. Epub 2016 Mar 3. Vaccine. 2016. PMID: 26945099
-
DNA vaccination against tumors.J Gene Med. 2005 Jan;7(1):3-17. doi: 10.1002/jgm.669. J Gene Med. 2005. PMID: 15538731 Review.
-
Justification for the inclusion of Gag in HIV vaccine candidates.Expert Rev Vaccines. 2016 May;15(5):585-98. doi: 10.1586/14760584.2016.1129904. Epub 2015 Dec 28. Expert Rev Vaccines. 2016. PMID: 26645951 Review.
Cited by
-
Expression Kinetics and Innate Immune Response after Electroporation and LNP-Mediated Delivery of a Self-Amplifying mRNA in the Skin.Mol Ther Nucleic Acids. 2019 Sep 6;17:867-878. doi: 10.1016/j.omtn.2019.08.001. Epub 2019 Aug 7. Mol Ther Nucleic Acids. 2019. PMID: 31472371 Free PMC article.
-
Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma.Cancers (Basel). 2022 Sep 17;14(18):4512. doi: 10.3390/cancers14184512. Cancers (Basel). 2022. PMID: 36139670 Free PMC article.
-
Intradermal DNA vaccination combined with dual CTLA-4 and PD-1 blockade provides robust tumor immunity in murine melanoma.PLoS One. 2019 May 31;14(5):e0217762. doi: 10.1371/journal.pone.0217762. eCollection 2019. PLoS One. 2019. PMID: 31150505 Free PMC article.
-
A Novel Vaccine Targeting Glypican-3 as a Treatment for Hepatocellular Carcinoma.Mol Ther. 2017 Oct 4;25(10):2299-2308. doi: 10.1016/j.ymthe.2017.08.005. Epub 2017 Aug 10. Mol Ther. 2017. PMID: 28865999 Free PMC article.
-
Nucleic Acid Immunotherapeutics for Cancer.ACS Appl Bio Mater. 2020 May 18;3(5):2838-2849. doi: 10.1021/acsabm.0c00101. Epub 2020 Mar 16. ACS Appl Bio Mater. 2020. PMID: 33681722 Free PMC article.
References
-
- Donnelly, JJ, Wahren, B and Liu, MA (2005). DNA vaccines: progress and challenges. J Immunol 175: 633–639. - PubMed
-
- Braumüller, H, Wieder, T, Brenner, E, Aßmann, S, Hahn, M, Alkhaled, M et al. (2013). T-helper-1-cell cytokines drive cancer into senescence. Nature 494: 361–365. - PubMed
-
- Rosenberg, SA, Yannelli, JR, Yang, JC, Topalian, SL, Schwartzentruber, DJ, Weber, JS et al. (1994). Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86: 1159–1166. - PubMed
-
- Castellino, F and Germain, RN (2006). Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol 24: 519–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

