Intratracheal Administration of Mesenchymal Stem Cells Modulates Tachykinin System, Suppresses Airway Remodeling and Reduces Airway Hyperresponsiveness in an Animal Model
- PMID: 27434719
- PMCID: PMC4951036
- DOI: 10.1371/journal.pone.0158746
Intratracheal Administration of Mesenchymal Stem Cells Modulates Tachykinin System, Suppresses Airway Remodeling and Reduces Airway Hyperresponsiveness in an Animal Model
Abstract
Background: The need for new options for chronic lung diseases promotes the research on stem cells for lung repair. Bone marrow-derived mesenchymal stem cells (MSCs) can modulate lung inflammation, but the data on cellular processes involved in early airway remodeling and the potential involvement of neuropeptides are scarce.
Objectives: To elucidate the mechanisms by which local administration of MSCs interferes with pathophysiological features of airway hyperresponsiveness in an animal model.
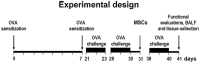
Methods: GFP-tagged mouse MSCs were intratracheally delivered in the ovalbumin mouse model with subsequent functional tests, the analysis of cytokine levels, neuropeptide expression and histological evaluation of MSCs fate and airway pathology. Additionally, MSCs were exposed to pro-inflammatory factors in vitro.
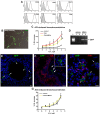
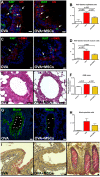
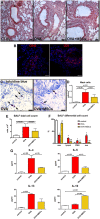
Results: Functional improvement was observed after MSC administration. Although MSCs did not adopt lung cell phenotypes, cell therapy positively affected airway remodeling reducing the hyperplastic phase of the gain in bronchial smooth muscle mass, decreasing the proliferation of epithelium in which mucus metaplasia was also lowered. Decrease of interleukin-4, interleukin-5, interleukin-13 and increase of interleukin-10 in bronchoalveolar lavage was also observed. Exposed to pro-inflammatory cytokines, MSCs upregulated indoleamine 2,3-dioxygenase. Moreover, asthma-related in vivo upregulation of pro-inflammatory neurokinin 1 and neurokinin 2 receptors was counteracted by MSCs that also determined a partial restoration of VIP, a neuropeptide with anti-inflammatory properties.
Conclusion: Intratracheally administered MSCs positively modulate airway remodeling, reduce inflammation and improve function, demonstrating their ability to promote tissue homeostasis in the course of experimental allergic asthma. Because of a limited tissue retention, the functional impact of MSCs may be attributed to their immunomodulatory response combined with the interference of neuropeptide system activation and tissue remodeling.
Conflict of interest statement
Figures
Similar articles
-
Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice.Am J Respir Cell Mol Biol. 2015 Nov;53(5):615-24. doi: 10.1165/rcmb.2014-0431OC. Am J Respir Cell Mol Biol. 2015. PMID: 25789608
-
New Role of Adult Lung c-kit+ Cells in a Mouse Model of Airway Hyperresponsiveness.Mediators Inflamm. 2016;2016:3917471. doi: 10.1155/2016/3917471. Epub 2016 Dec 20. Mediators Inflamm. 2016. PMID: 28090152 Free PMC article.
-
Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma.Stem Cells Transl Med. 2019 Mar;8(3):301-312. doi: 10.1002/sctm.18-0056. Epub 2018 Nov 13. Stem Cells Transl Med. 2019. PMID: 30426724 Free PMC article.
-
The role of neural inflammation in asthma and chronic obstructive pulmonary disease.Ann N Y Acad Sci. 2003 May;992:218-30. doi: 10.1111/j.1749-6632.2003.tb03152.x. Ann N Y Acad Sci. 2003. PMID: 12794061 Review.
-
Stem cells in animal asthma models: a systematic review.Cytotherapy. 2014 Dec;16(12):1629-42. doi: 10.1016/j.jcyt.2014.08.008. Epub 2014 Oct 18. Cytotherapy. 2014. PMID: 25442788
Cited by
-
The Usefulness of Mesenchymal Stem Cells beyond the Musculoskeletal System in Horses.Animals (Basel). 2021 Mar 25;11(4):931. doi: 10.3390/ani11040931. Animals (Basel). 2021. PMID: 33805967 Free PMC article. Review.
-
Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma.Stem Cells Transl Med. 2017 Jun;6(6):1557-1567. doi: 10.1002/sctm.16-0398. Epub 2017 Apr 20. Stem Cells Transl Med. 2017. PMID: 28425576 Free PMC article.
-
Nociceptin/Orphanin Fq in inflammation and remodeling of the small airways in experimental model of airway hyperresponsiveness.Physiol Rep. 2018 Oct;6(20):e13906. doi: 10.14814/phy2.13906. Physiol Rep. 2018. PMID: 30370666 Free PMC article.
-
Old Friends with Unexploited Perspectives: Current Advances in Mesenchymal Stem Cell-Based Therapies in Asthma.Stem Cell Rev Rep. 2021 Aug;17(4):1323-1342. doi: 10.1007/s12015-021-10137-7. Epub 2021 Mar 1. Stem Cell Rev Rep. 2021. PMID: 33649900 Free PMC article. Review.
-
Anti-Inflammatory Activity of a Peptide from Skipjack (Katsuwonus pelamis).Mar Drugs. 2019 Oct 13;17(10):582. doi: 10.3390/md17100582. Mar Drugs. 2019. PMID: 31614893 Free PMC article.
References
-
- Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 2005;116:477–486. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

