Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline
- PMID: 27435091
- PMCID: PMC4995362
- DOI: 10.1093/brain/aww180
Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline
Abstract
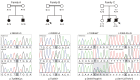
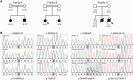
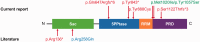
SYNJ1 encodes a polyphosphoinositide phosphatase, synaptojanin 1, which contains two consecutive phosphatase domains and plays a prominent role in synaptic vesicle dynamics. Autosomal recessive inherited variants in SYNJ1 have previously been associated with two different neurological diseases: a recurrent homozygous missense variant (p.Arg258Gln) that abolishes Sac1 phosphatase activity was identified in three independent families with early onset parkinsonism, whereas a homozygous nonsense variant (p.Arg136*) causing a severe decrease of mRNA transcript was found in a single patient with intractable epilepsy and tau pathology. We performed whole exome or genome sequencing in three independent sib pairs with early onset refractory seizures and progressive neurological decline, and identified novel segregating recessive SYNJ1 defects. A homozygous missense variant resulting in an amino acid substitution (p.Tyr888Cys) was found to impair, but not abolish, the dual phosphatase activity of SYNJ1, whereas three premature stop variants (homozygote p.Trp843* and compound heterozygote p.Gln647Argfs*6/p.Ser1122Thrfs*3) almost completely abolished mRNA transcript production. A genetic follow-up screening in a large cohort of 543 patients with a wide phenotypical range of epilepsies and intellectual disability revealed no additional pathogenic variants, showing that SYNJ1 deficiency is rare and probably linked to a specific phenotype. While variants leading to early onset parkinsonism selectively abolish Sac1 function, our results provide evidence that a critical reduction of the dual phosphatase activity of SYNJ1 underlies a severe disorder with neonatal refractory epilepsy and a neurodegenerative disease course. These findings further expand the clinical spectrum of synaptic dysregulation in patients with severe epilepsy, and emphasize the importance of this biological pathway in seizure pathophysiology.
Keywords: SYNJ1; SYNJ1 dual phosphatase activity; early onset epilepsy; neurodegenerative disorder; recessive disorder.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
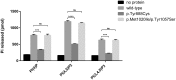

Similar articles
-
Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism.Hum Mutat. 2013 Sep;34(9):1208-15. doi: 10.1002/humu.22373. Epub 2013 Aug 6. Hum Mutat. 2013. PMID: 23804577
-
The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures.Hum Mutat. 2013 Sep;34(9):1200-7. doi: 10.1002/humu.22372. Epub 2013 Jul 19. Hum Mutat. 2013. PMID: 23804563 Free PMC article.
-
A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: a fertility application of whole exome sequencing.Hum Reprod. 2016 Apr;31(4):905-14. doi: 10.1093/humrep/dew025. Epub 2016 Feb 23. Hum Reprod. 2016. PMID: 26911863 Free PMC article.
-
A Rare Cause of Primary Microcephaly: 4 New Variants in CDK5RAP2 Gene and Review of the Literature.Am J Med Genet A. 2025 Sep;197(9):e64072. doi: 10.1002/ajmg.a.64072. Epub 2025 Apr 17. Am J Med Genet A. 2025. PMID: 40243280 Review.
-
Compound heterozygous ZNF335 variants in a patient with microcephaly, refractory epilepsy, and severe developmental delay: A case report and literature review.Congenit Anom (Kyoto). 2025 Jan-Dec;65(1):e70014. doi: 10.1111/cga.70014. Congenit Anom (Kyoto). 2025. PMID: 40583037 Review.
Cited by
-
Identification of SYNJ1 in a Complex Case of Juvenile Parkinsonism Using a Multiomics Approach.Int J Mol Sci. 2024 Sep 9;25(17):9754. doi: 10.3390/ijms25179754. Int J Mol Sci. 2024. PMID: 39273702 Free PMC article.
-
A Novel SYNJ1 Mutation in a Tunisian Family with Juvenile Parkinson's Disease Associated with Epilepsy.J Mol Neurosci. 2018 Oct;66(2):273-278. doi: 10.1007/s12031-018-1167-2. Epub 2018 Sep 5. J Mol Neurosci. 2018. PMID: 30187305
-
Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons.Neuron. 2017 Feb 22;93(4):882-896.e5. doi: 10.1016/j.neuron.2017.01.019. Neuron. 2017. PMID: 28231468 Free PMC article.
-
Temporal Vestibular Deficits in synaptojanin 1 (synj1) Mutants.Front Mol Neurosci. 2021 Jan 18;13:604189. doi: 10.3389/fnmol.2020.604189. eCollection 2020. Front Mol Neurosci. 2021. PMID: 33584199 Free PMC article.
-
Diverse Functions of Parkin in Midbrain Dopaminergic Neurons.Mov Disord. 2024 Aug;39(8):1282-1288. doi: 10.1002/mds.29890. Epub 2024 Jun 10. Mov Disord. 2024. PMID: 38858837 Free PMC article. Review.
References
-
- Brodin L, Low P, Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol 2000; 10: 312–20. - PubMed
-
- Cestra G, Castagnoli L, Dente L, Minenkova O, Petrelli A, Migone N, et al. The SH3 domains of endophilin and amphiphysin bind to the proline-rich region of synaptojanin 1 at distinct sites that display an unconventional binding specificity. J Biol Chem 1999; 274: 32001–7. - PubMed
-
- Cremona O, Di Paolo G, Wenk MR, Kim WT, Takei K, Daniell L, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 1999; 99: 179–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

