Identification of adducin-binding residues on the cytoplasmic domain of erythrocyte membrane protein, band 3
- PMID: 27435097
- PMCID: PMC5042879
- DOI: 10.1042/BCJ20160328
Identification of adducin-binding residues on the cytoplasmic domain of erythrocyte membrane protein, band 3
Abstract
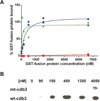
Two major complexes form structural bridges that connect the erythrocyte membrane to its underlying spectrin-based cytoskeleton. Although the band 3-ankyrin bridge may account for most of the membrane-to-cytoskeleton interactions, the linkage between the cytoplasmic domain of band 3 (cdb3) and adducin has also been shown to be critical to membrane integrity. In the present paper, we demonstrate that adducin, a major component of the spectrin-actin junctional complex, binds primarily to residues 246-264 of cdb3, and mutation of two exposed glutamic acid residues within this sequence completely abrogates both α- and β-adducin binding. Because these residues are located next to the ankyrin-binding site on cdb3, it seems unlikely that band 3 can bind ankyrin and adducin concurrently, reducing the chances of an association between the ankyrin and junctional complexes that would significantly compromise erythrocyte membrane integrity. We also demonstrate that adducin binds the kidney isoform of cdb3, a spliceoform that lacks the first 65 amino acids of erythrocyte cdb3, including the central strand of a large β-pleated sheet. Because kidney cdb3 is not known to bind any of the common peripheral protein partners of erythrocyte cdb3, including ankyrin, protein 4.1, glyceraldehyde-3-phosphate dehydrogenase, aldolase, and phosphofructokinase, retention of this affinity for adducin was unexpected.
Keywords: adducin; anion exchanger 1; cytoplasmic domain of band 3; erythrocytes; junctional complexes; membrane structure.
© 2016 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
DECLARATIONS OF INTEREST The authors declare that they have no conflicts of interest with the contents of this article.
Figures





Similar articles
-
Identification of contact sites between ankyrin and band 3 in the human erythrocyte membrane.Biochemistry. 2012 Aug 28;51(34):6838-46. doi: 10.1021/bi300693k. Epub 2012 Aug 14. Biochemistry. 2012. PMID: 22861190 Free PMC article.
-
Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis.J Biol Chem. 2003 Feb 28;278(9):6879-84. doi: 10.1074/jbc.M211137200. Epub 2002 Dec 12. J Biol Chem. 2003. PMID: 12482869
-
Partial characterization of the cytoplasmic domain of human kidney band 3.J Biol Chem. 1995 Jul 28;270(30):17892-7. doi: 10.1074/jbc.270.30.17892. J Biol Chem. 1995. PMID: 7629093
-
The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life.Protoplasma. 2010 Aug;244(1-4):99-131. doi: 10.1007/s00709-010-0181-1. Epub 2010 Jul 29. Protoplasma. 2010. PMID: 20668894 Review.
-
The molecular basis for membrane - cytoskeleton association in human erythrocytes.J Cell Biochem. 1982;18(1):49-65. doi: 10.1002/jcb.1982.240180106. J Cell Biochem. 1982. PMID: 6461664 Review.
Cited by
-
Red cell membrane proteins.Hemasphere. 2019 Jun 30;3(Suppl):154-156. doi: 10.1097/HS9.0000000000000189. eCollection 2019 Jun. Hemasphere. 2019. PMID: 35309774 Free PMC article. No abstract available.
-
Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1.Am J Physiol Cell Physiol. 2021 Dec 1;321(6):C1028-C1059. doi: 10.1152/ajpcell.00275.2021. Epub 2021 Oct 20. Am J Physiol Cell Physiol. 2021. PMID: 34669510 Free PMC article. Review.
-
NIMA-related kinase 1 (NEK1) regulates meiosis I spindle assembly by altering the balance between α-Adducin and Myosin X.PLoS One. 2017 Oct 5;12(10):e0185780. doi: 10.1371/journal.pone.0185780. eCollection 2017. PLoS One. 2017. PMID: 28982183 Free PMC article.
-
Syk inhibitors interfere with erythrocyte membrane modification during P falciparum growth and suppress parasite egress.Blood. 2017 Aug 24;130(8):1031-1040. doi: 10.1182/blood-2016-11-748053. Epub 2017 Jun 20. Blood. 2017. PMID: 28634183 Free PMC article.
References
-
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods in enzymology. 1983;96:313–324. - PubMed
-
- Lux SE, Tse WT, Menninger JC, John KM, Harris P, Shalev O, Chilcote RR, Marchesi SL, Watkins PC, Bennett V, et al. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990;345:736–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

