Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease
- PMID: 27435107
- PMCID: PMC5250616
- DOI: 10.1038/mi.2016.63
Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease
Abstract
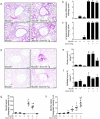
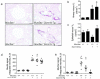
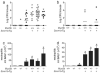
Airway diseases, including cigarette smoke-induced chronic bronchitis, cystic fibrosis, and primary ciliary dyskinesia are associated with decreased mucociliary clearance (MCC). However, it is not known whether a simple reduction in MCC or concentration-dependent mucus adhesion to airway surfaces dominates disease pathogenesis or whether decreasing the concentration of secreted mucins may be therapeutic. To address these questions, Scnn1b-Tg mice, which exhibit airway mucus dehydration/adhesion, were compared and crossed with Muc5b- and Muc5ac-deficient mice. Absence of Muc5b caused a 90% reduction in MCC, whereas Scnn1b-Tg mice exhibited an ∼50% reduction. However, the degree of MCC reduction did not correlate with bronchitic airway pathology, which was observed only in Scnn1b-Tg mice. Ablation of Muc5b significantly reduced the extent of mucus plugging in Scnn1b-Tg mice. However, complete absence of Muc5b in Scnn1b-Tg mice was associated with increased airway inflammation, suggesting that Muc5b is required to maintain immune homeostasis. Loss of Muc5ac had few phenotypic consequences in Scnn1b-Tg mice. These data suggest that: (i) mucus hyperconcentration dominates over MCC reduction alone to produce bronchitic airway pathology; (ii) Muc5b is the dominant contributor to the Scnn1b-Tg phenotype; and (iii) therapies that limit mucin secretion may reduce plugging, but complete Muc5b removal from airway surfaces may be detrimental.
Figures





Similar articles
-
Role of Spdef in the Regulation of Muc5b Expression in the Airways of Naive and Mucoobstructed Mice.Am J Respir Cell Mol Biol. 2018 Sep;59(3):383-396. doi: 10.1165/rcmb.2017-0127OC. Am J Respir Cell Mol Biol. 2018. PMID: 29579396 Free PMC article.
-
Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease.Mucosal Immunol. 2017 May;10(3):829. doi: 10.1038/mi.2017.29. Mucosal Immunol. 2017. PMID: 28435155 No abstract available.
-
Ablation of IL-33 Suppresses Th2 Responses but Is Accompanied by Sustained Mucus Obstruction in the Scnn1b Transgenic Mouse Model.J Immunol. 2020 Mar 15;204(6):1650-1660. doi: 10.4049/jimmunol.1900234. Epub 2020 Feb 14. J Immunol. 2020. PMID: 32060135 Free PMC article.
-
Immunopathology of Airway Surface Liquid Dehydration Disease.J Immunol Res. 2019 Jul 14;2019:2180409. doi: 10.1155/2019/2180409. eCollection 2019. J Immunol Res. 2019. PMID: 31396541 Free PMC article. Review.
-
Mucus hypersecretion in asthma: causes and effects.Curr Opin Pulm Med. 2009 Jan;15(1):4-11. doi: 10.1097/MCP.0b013e32831da8d3. Curr Opin Pulm Med. 2009. PMID: 19077699 Free PMC article. Review.
Cited by
-
Lack of Kcnn4 improves mucociliary clearance in muco-obstructive lung disease.JCI Insight. 2020 Aug 20;5(16):e140076. doi: 10.1172/jci.insight.140076. JCI Insight. 2020. PMID: 32814712 Free PMC article.
-
Airway epithelial cell-specific deletion of HMGB1 exaggerates inflammatory responses in mice with muco-obstructive airway disease.Front Immunol. 2023 Jan 19;13:944772. doi: 10.3389/fimmu.2022.944772. eCollection 2022. Front Immunol. 2023. PMID: 36741411 Free PMC article.
-
Gene expression analysis of Atlantic salmon gills reveals mucin 5 and interleukin 4/13 as key molecules during amoebic gill disease.Sci Rep. 2018 Sep 12;8(1):13689. doi: 10.1038/s41598-018-32019-8. Sci Rep. 2018. PMID: 30209326 Free PMC article.
-
Global warming risks dehydrating and inflaming human airways.Commun Earth Environ. 2025;6(1):193. doi: 10.1038/s43247-025-02161-z. Epub 2025 Mar 17. Commun Earth Environ. 2025. PMID: 40678270 Free PMC article.
-
Muc5b-deficient mice develop early histological lung abnormalities.Biol Open. 2019 Nov 7;8(11):bio046359. doi: 10.1242/bio.046359. Biol Open. 2019. PMID: 31699684 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P50 HL060280/HL/NHLBI NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- P01 HL110873/HL/NHLBI NIH HHS/United States
- P50 HL107168/HL/NHLBI NIH HHS/United States
- R21 ES023384/ES/NIEHS NIH HHS/United States
- R01 HL080396/HL/NHLBI NIH HHS/United States
- UH3 HL123645/HL/NHLBI NIH HHS/United States
- P50 HL084934/HL/NHLBI NIH HHS/United States
- UH2 HL123645/HL/NHLBI NIH HHS/United States
- R01 HL130938/HL/NHLBI NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

