Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis
- PMID: 27435108
- PMCID: PMC5250615
- DOI: 10.1038/mi.2016.61
Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis
Abstract
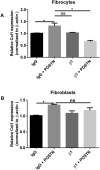
Fibrocytes are circulating mesenchymal precursors (CD45+, col 1+) recruited to fibrotic areas. Fibrocytes secrete profibrotic mediators including periostin; a matricellular protein that regulates cellular interactions with extracellular matrix (ECM) components. In bleomycin-induced fibrosis, periostin deficiency in structural or hematopoietic cells limits development of pulmonary fibrosis. To determine if hematopoietic-derived fibrocytes might secrete soluble factors to activate structural myofibroblast differentiation, wild-type (WT) fibroblasts were treated with conditioned medium from fibrocytes isolated from bleomycin-treated WT or periostin-/- mice. After 24 h we saw less α-smooth muscle actin expression in cells treated with conditioned medium from periostin-/- fibrocytes. Adoptive transfer of WT fibrocytes augmented lung fibrosis to a greater extent than transfer of fibrocytes from periostin-/- mice. In vitro analysis of fibrocytes and fibroblasts isolated from WT and periostin-/- mice treated with TGFβ1 or periostin demonstrated co-regulation of mesenchymal activation and beta 1 integrin as a potential receptor for periostin on fibrocytes. Additionally, connective tissue growth factor (CTGF) mRNA expression was increased in fibrocytes treated with periostin whereas CTGF and lysl oxidase (LOX) mRNA expression was low in bleomycin-treated periostin-/- fibrocytes. These data suggest fibrocytes may augment bleomycin-induced fibrosis via secretion of periostin and other soluble factors that promote myofibroblast differentiation.
Figures








References
-
- King TE, Jr., Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. - PubMed
-
- Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11(8):1176–1185. - PubMed
-
- Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr., et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12):963–967. Pt 1. - PubMed
-
- Antoniou KM, Pataka A, Bouros D, Siafakas NM. Pathogenetic pathways and novel pharmacotherapeutic targets in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2007;20(5):453–461. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

