Safety of using real-time sensor glucose values for treatment decisions in adolescents with poorly controlled type 1 diabetes mellitus: a pilot study
- PMID: 27435145
- PMCID: PMC5250611
- DOI: 10.1111/pedi.12404
Safety of using real-time sensor glucose values for treatment decisions in adolescents with poorly controlled type 1 diabetes mellitus: a pilot study
Abstract
Background: This study explored the safety of using real-time sensor glucose (SG) data for treatment decisions in adolescents with poorly controlled type 1 diabetes.
Methods: Ten adolescents with type 1 diabetes, HbA1c ≥9% on insulin pumps were admitted to the clinical research center and a continuous glucose sensor was inserted. Plasma glucose was measured at least hourly using Yellow Springs Instrument's (YSI) glucose analyzer. Starting at dinner, SG rather than YSI was used for treatment decisions unless YSI was <70 mg/dL (<3.9 mmol/L) or specific criteria indicating SG and YSI were very discordant were met. Participants were discharged after lunch the next day.
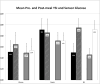
Results: Ten participants (seven males; 15.2-17.8 year old) completed the study. The range of differences between high glucose correction doses using SG vs YSI for calculations was -2 (SG < YSI dose) to +1 (SG > YSI dose); this difference was two units in only 2 of 23 correction doses given (all SG < YSI dose). There were five episodes of mild hypoglycemia in two patients, two of which occurred after using SG for dose calculations. There was no severe hypoglycemia and no YSI glucose >350 mg/dL (19.4 mmol/L). Mean (±SE) pre- and postmeal YSI glucose were 163 ± 11 and 183 ± 12 mg/dL (9.1 ± 0.6 and 10.2 ± 0.7 mmol/L), respectively.
Conclusion: Use of real-time continuous glucose monitoring for treatment decisions was safe and did not result in significant over- or undertreatment. Use of SG for treatment decisions under supervised inpatient conditions is a suitable alternative to repeated fingerstick glucose monitoring. Outpatient studies using SG in real-time are needed.
Trial registration: ClinicalTrials.gov NCT01586065.
Keywords: continuous glucose monitoring; pediatrics; type 1 diabetes.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Author Disclosure Statement.
EB, KE and JH report no competing financial interests exist.
Figures
Similar articles
-
Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study.Diabetes Technol Ther. 2012 Mar;14(3):205-9. doi: 10.1089/dia.2011.0292. Epub 2012 Feb 8. Diabetes Technol Ther. 2012. PMID: 22316089 Clinical Trial.
-
Evaluation of a Predictive Low-Glucose Management System In-Clinic.Diabetes Technol Ther. 2017 May;19(5):288-292. doi: 10.1089/dia.2016.0319. Epub 2017 Feb 16. Diabetes Technol Ther. 2017. PMID: 28221823
-
Reduction in Hypoglycemia With the Predictive Low-Glucose Management System: A Long-term Randomized Controlled Trial in Adolescents With Type 1 Diabetes.Diabetes Care. 2018 Feb;41(2):303-310. doi: 10.2337/dc17-1604. Epub 2017 Nov 30. Diabetes Care. 2018. PMID: 29191844 Clinical Trial.
-
The efficacy and safety of insulin pump therapy with predictive low glucose suspend feature in decreasing hypoglycemia in children with type 1 diabetes mellitus: A systematic review and meta-analysis.Pediatr Diabetes. 2020 Nov;21(7):1256-1267. doi: 10.1111/pedi.13088. Epub 2020 Sep 14. Pediatr Diabetes. 2020. PMID: 32738022
-
Continuous subcutaneous insulin infusion: Special needs for children.Pediatr Diabetes. 2017 Jun;18(4):255-261. doi: 10.1111/pedi.12491. Epub 2017 Apr 20. Pediatr Diabetes. 2017. PMID: 28425167 Review.
Cited by
-
Ambulatory glucose profile analysis of the juvenile diabetes research foundation continuous glucose monitoring dataset-Applications to the pediatric diabetes population.Pediatr Diabetes. 2017 Nov;18(7):622-628. doi: 10.1111/pedi.12474. Epub 2016 Nov 23. Pediatr Diabetes. 2017. PMID: 27878929 Free PMC article.
-
Evidence from clinical trials on high-risk medical devices in children: a scoping review.Pediatr Res. 2024 Feb;95(3):615-624. doi: 10.1038/s41390-023-02819-4. Epub 2023 Sep 28. Pediatr Res. 2024. PMID: 37758865 Free PMC article.
-
Technologies for Diabetes Self-Monitoring: A Scoping Review and Assessment Using the REASSURED Criteria.J Diabetes Sci Technol. 2022 Jul;16(4):962-970. doi: 10.1177/1932296821997909. Epub 2021 Mar 9. J Diabetes Sci Technol. 2022. PMID: 33686875 Free PMC article.
References
-
- Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine. 2013;43:41–50. - PubMed
-
- Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, et al. FreeStyle navigator continuous glucose monitoring system use in children with type 1 diabetes using glargine-based multiple daily dose regimens: results of a pilot trial Diabetes Research in Children Network (DirecNet) Study Group. Diabetes Care. 2008;31:525–7. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

