Aerobic exercise training protects against endothelial dysfunction by increasing nitric oxide and hydrogen peroxide production in LDL receptor-deficient mice
- PMID: 27435231
- PMCID: PMC4950099
- DOI: 10.1186/s12967-016-0972-z
Aerobic exercise training protects against endothelial dysfunction by increasing nitric oxide and hydrogen peroxide production in LDL receptor-deficient mice
Abstract
Background: Endothelial dysfunction associated with hypercholesterolemia is an early event in atherosclerosis characterized by redox imbalance associated with high superoxide production and reduced nitric oxide (NO) and hydrogen peroxide (H2O2) production. Aerobic exercise training (AET) has been demonstrated to ameliorate atherosclerotic lesions and oxidative stress in advanced atherosclerosis. However, whether AET protects against the early mechanisms of endothelial dysfunction in familial hypercholesterolemia remains unclear. This study investigated the effects of AET on endothelial dysfunction and vascular redox status in the aortas of LDL receptor knockout mice (LDLr(-/-)), a genetic model of familial hypercholesterolemia.
Methods: Twelve-week-old C57BL/6J (WT) and LDLr(-/-) mice were divided into sedentary and exercised (AET on a treadmill 1 h/5 × per week) groups for 4 weeks. Changes in lipid profiles, endothelial function, and aortic NO, H2O2 and superoxide production were examined.
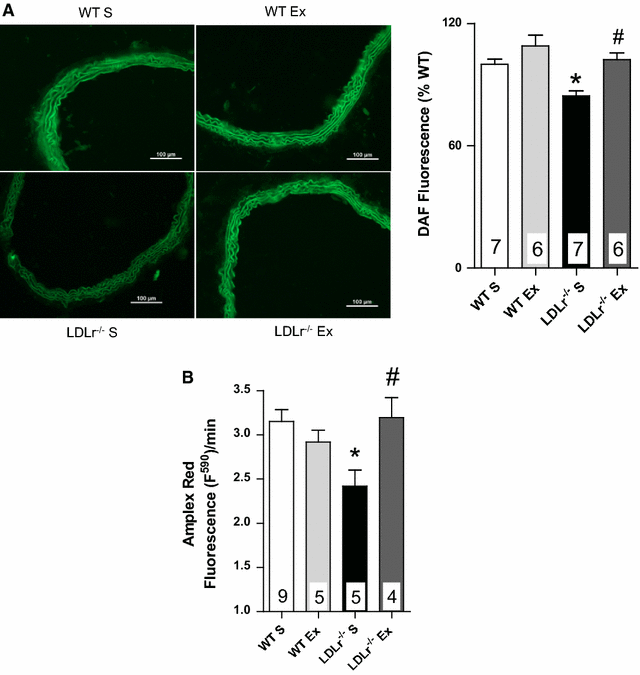
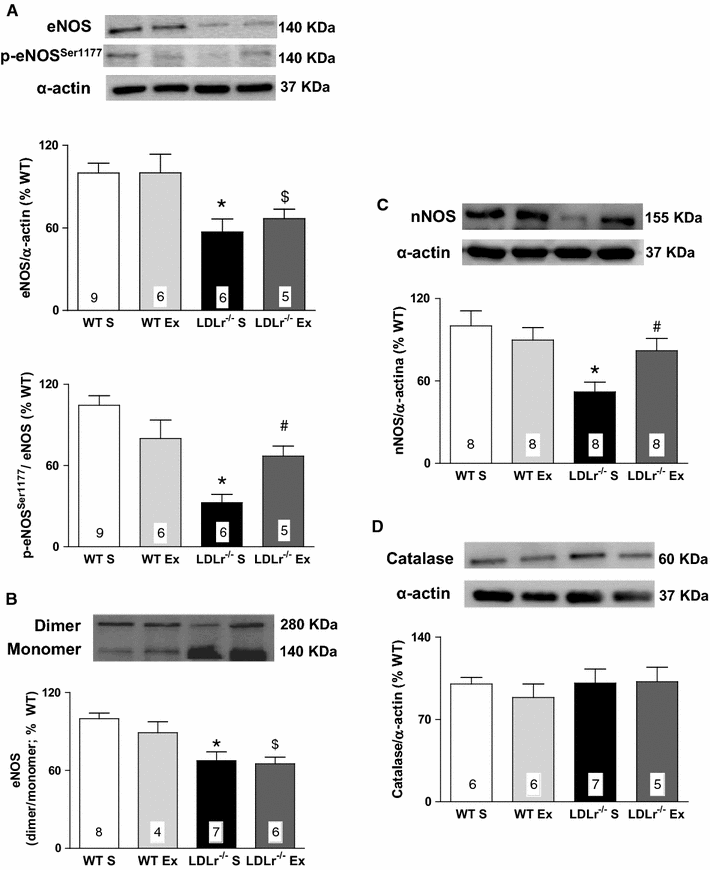
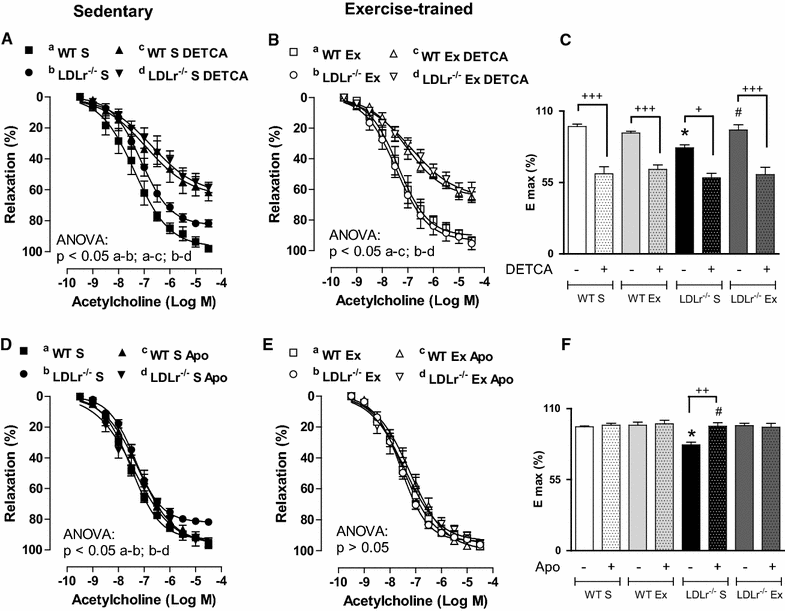
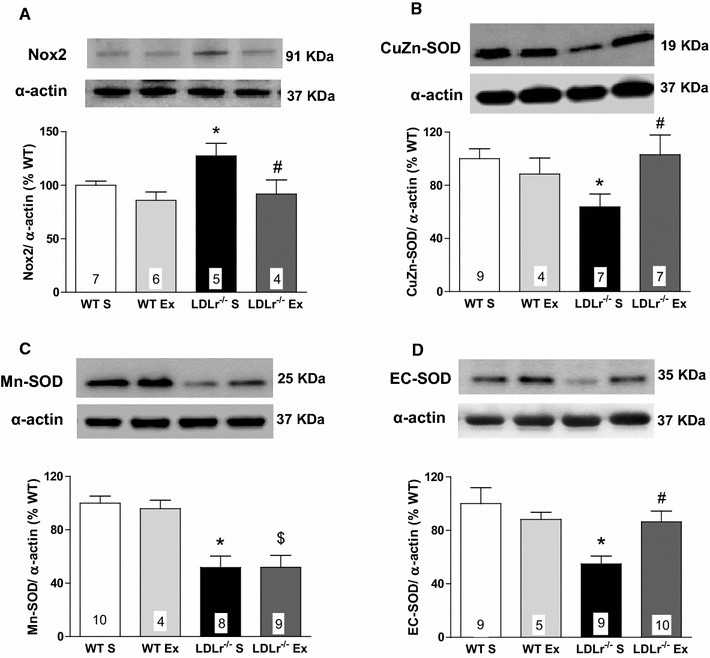
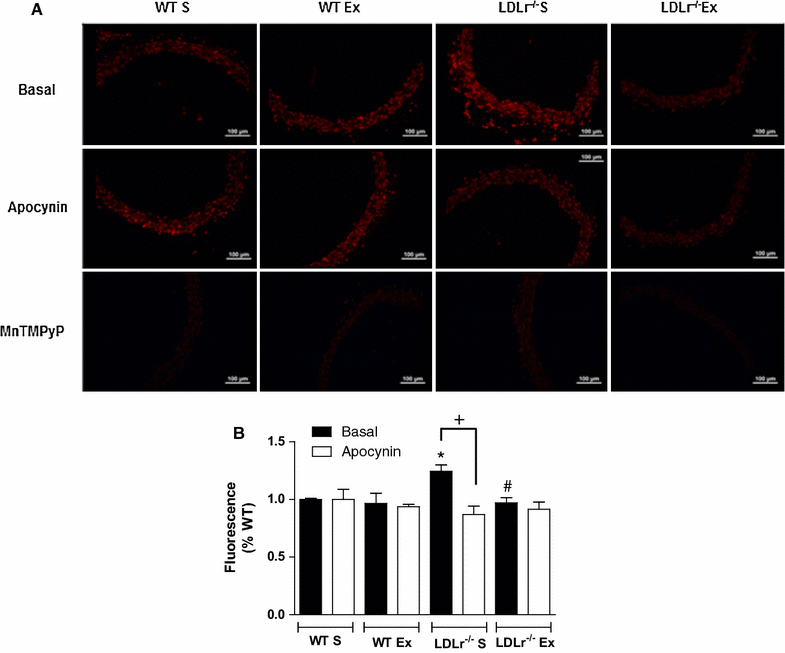
Results: Total cholesterol and triglycerides were increased in sedentary and exercised LDLr(-/-) mice. Endothelium-dependent relaxation induced by acetylcholine was impaired in aortas of sedentary LDLr(-/-) mice but not in the exercised group. Inhibition of NO synthase (NOS) activity or H2O2 decomposition by catalase abolished the differences in the acetylcholine response between the animals. No changes were noted in the relaxation response induced by NO donor sodium nitroprusside or H2O2. Neuronal NOS expression and endothelial NOS phosphorylation (Ser1177), as well as NO and H2O2 production, were reduced in aortas of sedentary LDLr(-/-) mice and restored by AET. Incubation with apocynin increased acetylcholine-induced relaxation in sedentary, but not exercised LDLr(-/-) mice, suggesting a minor participation of NADPH oxidase in the endothelium-dependent relaxation after AET. Consistent with these findings, Nox2 expression and superoxide production were reduced in the aortas of exercised compared to sedentary LDLr(-/-) mice. Furthermore, the aortas of sedentary LDLr(-/-) mice showed reduced expression of superoxide dismutase (SOD) isoforms and minor participation of Cu/Zn-dependent SODs in acetylcholine-induced, endothelium-dependent relaxation, abnormalities that were partially attenuated in exercised LDLr(-/-) mice.
Conclusion: The data gathered by this study suggest AET as a potential non-pharmacological therapy in the prevention of very early endothelial dysfunction and redox imbalance in familial hypercholesterolemia via increases in NO bioavailability and H2O2 production.
Keywords: Aerobic exercise training; Endothelial dysfunction; Familial hypercholesterolemia; Hydrogen peroxide; LDL receptor-deficient mice; Nitric oxide synthase; Superoxide dismutase.
Figures


Similar articles
-
Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2.Br J Pharmacol. 2003 Apr;138(7):1215-20. doi: 10.1038/sj.bjp.0705164. Br J Pharmacol. 2003. PMID: 12711621 Free PMC article.
-
Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice.Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2333-40. doi: 10.1161/01.atv.17.11.2333. Arterioscler Thromb Vasc Biol. 1997. PMID: 9409199
-
Effects of dietary sodium on reactive oxygen species formation and endothelial dysfunction in low-density lipoprotein receptor-deficient mice on high-fat diet.Heart Vessels. 2008 Nov;23(6):420-9. doi: 10.1007/s00380-008-1066-5. Epub 2008 Nov 27. Heart Vessels. 2008. PMID: 19037590
-
Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis.Free Radic Biol Med. 2017 Aug;109:4-10. doi: 10.1016/j.freeradbiomed.2016.12.019. Epub 2016 Dec 14. Free Radic Biol Med. 2017. PMID: 27988339 Review.
-
The physiology and pathophysiology of the nitric oxide/superoxide system.Herz. 1997 Jun;22(3):158-72. doi: 10.1007/BF03044353. Herz. 1997. PMID: 9232165 Review.
Cited by
-
High-intensity exercise in hypoxia improves endothelial function via increased nitric oxide bioavailability in C57BL/6 mice.Acta Physiol (Oxf). 2021 Oct;233(2):e13700. doi: 10.1111/apha.13700. Epub 2021 Jun 19. Acta Physiol (Oxf). 2021. PMID: 34089562 Free PMC article.
-
Preclinical techniques to investigate exercise training in vascular pathophysiology.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1566-H1600. doi: 10.1152/ajpheart.00719.2020. Epub 2021 Jan 1. Am J Physiol Heart Circ Physiol. 2021. PMID: 33385323 Free PMC article.
-
Rationale and Design of the PARTNER Trial: Partnered Rhythmic Rehabilitation for Enhanced Motor-Cognition in Prodromal Alzheimer's Disease.J Alzheimers Dis. 2023;91(3):1019-1033. doi: 10.3233/JAD-220783. J Alzheimers Dis. 2023. PMID: 36530084 Free PMC article. Clinical Trial.
-
The Anti-atherogenic Role of Exercise Is Associated With the Attenuation of Bone Marrow-Derived Macrophage Activation and Migration in Hypercholesterolemic Mice.Front Physiol. 2020 Nov 23;11:599379. doi: 10.3389/fphys.2020.599379. eCollection 2020. Front Physiol. 2020. PMID: 33329050 Free PMC article.
-
Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy.Nitric Oxide. 2023 Feb 1;131:8-17. doi: 10.1016/j.niox.2022.11.006. Epub 2022 Dec 5. Nitric Oxide. 2023. PMID: 36470373 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

