IL-21 Enhances Natural Killer Cell Response to Cetuximab-Coated Pancreatic Tumor Cells
- PMID: 27435400
- PMCID: PMC5241232
- DOI: 10.1158/1078-0432.CCR-16-0004
IL-21 Enhances Natural Killer Cell Response to Cetuximab-Coated Pancreatic Tumor Cells
Abstract
Purpose: Alternative strategies to EGFR blockage by mAbs is necessary to improve the efficacy of therapy in patients with locally advanced or metastatic pancreatic cancer. One such strategy includes the use of NK cells to clear cetuximab-coated tumor cells, as need for novel therapeutic approaches to enhance the efficacy of cetuximab is evident. We show that IL-21 enhances NK cell-mediated effector functions against cetuximab-coated pancreatic tumor cells irrespective of KRAS mutation status.
Experimental design: NK cells from normal donors or donors with pancreatic cancer were used to assess ADCC, IFN-γ release, and T-cell chemotaxis toward human pancreatic cancer cell lines. The in vivo efficacy of IL-21 in combination with cetuximab was evaluated in a subcutaneous and intraperitoneal model of pancreatic cancer.
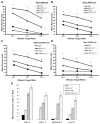
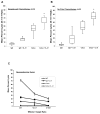
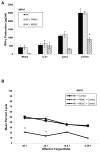
Results: NK cell lysis of cetuximab-coated wild-type and mutant kras pancreatic cancer cell lines were significantly higher following NK cell IL-21 treatment. In response to cetuximab-coated pancreatic tumor cells, IL-21-treated NK cells secreted significantly higher levels of IFN-γ and chemokines, increased chemotaxis of T cells, and enhanced NK cell signal transduction via activation of ERK and STAT1. Treatment of mice bearing subcutaneous or intraperitoneal EGFR-positive pancreatic tumor xenografts with mIL-21 and cetuximab led to significant inhibition of tumor growth, a result further enhanced by the addition of gemcitabine.
Conclusions: These results suggest that cetuximab treatment in combination with IL-21 adjuvant therapy in patients with EGFR-positive pancreatic cancer results in significant NK cell activation, irrespective of KRAS mutation status, and may be a potential therapeutic strategy. Clin Cancer Res; 23(2); 489-502. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




Similar articles
-
The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines.Clin Cancer Res. 2007 Nov 1;13(21):6419-28. doi: 10.1158/1078-0432.CCR-07-0865. Epub 2007 Oct 25. Clin Cancer Res. 2007. PMID: 17962339
-
Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines.Surgery. 2012 Sep;152(3):431-40. doi: 10.1016/j.surg.2012.05.035. Epub 2012 Jul 6. Surgery. 2012. PMID: 22770960 Free PMC article.
-
Interleukin-21 enhances NK cell activation in response to antibody-coated targets.J Immunol. 2006 Jul 1;177(1):120-9. doi: 10.4049/jimmunol.177.1.120. J Immunol. 2006. PMID: 16785506
-
NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis.Semin Cancer Biol. 2018 Dec;53:178-188. doi: 10.1016/j.semcancer.2018.08.001. Epub 2018 Aug 3. Semin Cancer Biol. 2018. PMID: 30081230 Review.
-
Curcumin and omega-3 fatty acids enhance NK cell-induced apoptosis of pancreatic cancer cells but curcumin inhibits interferon-γ production: benefits of omega-3 with curcumin against cancer.Molecules. 2015 Feb 12;20(2):3020-6. doi: 10.3390/molecules20023020. Molecules. 2015. PMID: 25685909 Free PMC article. Review.
Cited by
-
SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells.Oncotarget. 2017 Jun 21;8(32):53518-53530. doi: 10.18632/oncotarget.18591. eCollection 2017 Aug 8. Oncotarget. 2017. PMID: 28881828 Free PMC article.
-
From bench to bedside: the past, present and future of IL-21 immunotherapy.Med Oncol. 2024 Jun 20;41(7):181. doi: 10.1007/s12032-024-02404-7. Med Oncol. 2024. PMID: 38900341 Review.
-
Cellular senescence in ageing: from mechanisms to therapeutic opportunities.Nat Rev Mol Cell Biol. 2021 Feb;22(2):75-95. doi: 10.1038/s41580-020-00314-w. Epub 2020 Dec 16. Nat Rev Mol Cell Biol. 2021. PMID: 33328614 Free PMC article. Review.
-
The application of Interleukin-2 family cytokines in tumor immunotherapy research.Front Immunol. 2023 Mar 2;14:1090311. doi: 10.3389/fimmu.2023.1090311. eCollection 2023. Front Immunol. 2023. PMID: 36936961 Free PMC article. Review.
-
Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function.Clin Cancer Res. 2018 Apr 15;24(8):1891-1904. doi: 10.1158/1078-0432.CCR-17-0691. Epub 2018 Jan 23. Clin Cancer Res. 2018. PMID: 29363526 Free PMC article.
References
-
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. - PubMed
-
- Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23(1–2):74–9. - PubMed
-
- Hemming AW, Davis NL, Kluftinger A, Robinson B, Quenville NF, Liseman B, et al. Prognostic markers of colorectal cancer: an evaluation of DNA content, epidermal growth factor receptor, and Ki-67. J Surg Oncol. 1992;51(3):147–52. - PubMed
-
- Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–60. - PubMed
-
- Fan Z, Shang BY, Lu Y, Chou JL, Mendelsohn J. Reciprocal changes in p27(Kip1) and p21(Cip1) in growth inhibition mediated by blockade or overstimulation of epidermal growth factor receptors. Clin Cancer Res. 1997;3(11):1943–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

