Recovery from volumetric muscle loss injury: A comparison between young and aged rats
- PMID: 27435497
- PMCID: PMC5007166
- DOI: 10.1016/j.exger.2016.07.008
Recovery from volumetric muscle loss injury: A comparison between young and aged rats
Abstract
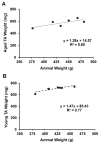
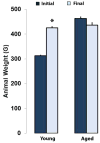
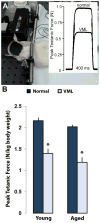
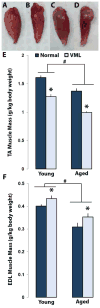
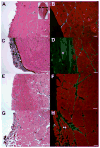
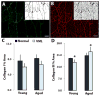
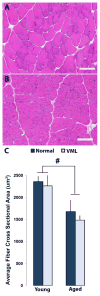
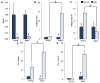
Termed volumetric muscle loss (VML), the bulk loss of skeletal muscle tissue either through trauma or surgery overwhelms the capacity for repair, leading to the formation of non-contractile scar tissue. The myogenic potential, along with other factors that influence wound repair are known to decline with age. In order to develop effective treatment strategies for VML injuries that are effective across a broad range of patient populations, it is necessary to understand how the response to VML injury is affected by aging. Towards this end, this study was conducted to compare the response of young and aged animal groups to a lower extremity VML injury. Young (3months, n=12) and aged (18months, n=8) male Fischer 344 rats underwent surgical VML injury of the tibialis anterior muscle. Three months after VML injury it was found that young TA muscle was on average 16% heavier than aged muscle when no VML injury was performed and 25% heavier when comparing VML treated young and aged animals (p<0.0001, p<0.0001). Peak contractile force for both the young and aged groups was found to decrease significantly following VML injury, producing 65% and 59% of the contralateral limbs' peak force, respectively (p<0.0001). However, there were no differences found for peak contractile force based on age, suggesting that VML affects muscle's ability to repair, regardless of age. In this study, we used the ratio of collagen I to MyoD expression as a metric for fibrosis vs. myogenesis. Decreasing fiber cross-sectional area with advancing age (p<0.005) coupled with the ratio of collagen I to MyoD expression, which increased with age, supports the thought that regeneration is impaired in the aged population in favor of fibrosis (p=0.0241). This impairment is also exacerbated by the contribution of VML injury, where a 77-fold increase in the ratio of collagen I to MyoD was observed in the aged group (p<0.0002). The aged animal model described in this study provides a tool for investigators exploring not only the development of VML injury strategies but also the effect of aging on muscle regeneration.
Keywords: Aging; Animal model; Musculoskeletal; Orthopedics; Tibialis anterior; VML.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Regenerative Repair of Volumetric Muscle Loss Injury is Sensitive to Age.Tissue Eng Part A. 2020 Jan;26(1-2):3-14. doi: 10.1089/ten.TEA.2019.0034. Epub 2019 Aug 9. Tissue Eng Part A. 2020. PMID: 31064280 Free PMC article.
-
Nandrolone supplementation does not improve functional recovery in an aged animal model of volumetric muscle loss injury.J Tissue Eng Regen Med. 2022 Apr;16(4):367-379. doi: 10.1002/term.3286. Epub 2022 Feb 3. J Tissue Eng Regen Med. 2022. PMID: 35113494
-
Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model.Tissue Eng Part A. 2016 Oct;22(19-20):1151-1163. doi: 10.1089/ten.TEA.2016.0134. Epub 2016 Sep 23. Tissue Eng Part A. 2016. PMID: 27570911 Free PMC article.
-
Pathophysiology of Volumetric Muscle Loss Injury.Cells Tissues Organs. 2016;202(3-4):180-188. doi: 10.1159/000443925. Epub 2016 Nov 9. Cells Tissues Organs. 2016. PMID: 27825160 Review.
-
Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review.Vet Surg. 2018 May;47(4):524-535. doi: 10.1111/vsu.12787. Epub 2018 Mar 30. Vet Surg. 2018. PMID: 29603757 Review.
Cited by
-
The effect of autologous repair and voluntary wheel running on force recovery in a rat model of volumetric muscle loss.Exp Physiol. 2021 Apr;106(4):994-1004. doi: 10.1113/EP089207. Epub 2021 Mar 2. Exp Physiol. 2021. PMID: 33600045 Free PMC article.
-
Local IL-10 delivery modulates the immune response and enhances repair of volumetric muscle loss muscle injury.Sci Rep. 2023 Feb 3;13(1):1983. doi: 10.1038/s41598-023-27981-x. Sci Rep. 2023. PMID: 36737628 Free PMC article.
-
Enhancing volumetric muscle loss (VML) recovery in a rat model using super durable hydrogels derived from bacteria.Bioact Mater. 2024 Jun 1;38:540-558. doi: 10.1016/j.bioactmat.2024.04.006. eCollection 2024 Aug. Bioact Mater. 2024. PMID: 38872731 Free PMC article.
-
Extracellular Matrix From Decellularized Wharton's Jelly Improves the Behavior of Cells From Degenerated Intervertebral Disc.Front Bioeng Biotechnol. 2020 Mar 27;8:262. doi: 10.3389/fbioe.2020.00262. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32292779 Free PMC article.
-
Age-associated functional healing of musculoskeletal trauma through regenerative engineering and rehabilitation.Biomater Sci. 2024 Oct 8;12(20):5186-5202. doi: 10.1039/d4bm00616j. Biomater Sci. 2024. PMID: 39172120
References
-
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–669. - PubMed
-
- Andreollo NA, Santos EF, Araujo MR, Lopes LR. Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig. 2012;25:49–51. - PubMed
-
- Aurora A, Roe JL, Corona BT, Walters TJ. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials. 2015;67:393–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

