Cryptic protein-protein interaction motifs in the cytoplasmic domain of MHCI proteins
- PMID: 27435737
- PMCID: PMC4950430
- DOI: 10.1186/s12865-016-0154-z
Cryptic protein-protein interaction motifs in the cytoplasmic domain of MHCI proteins
Abstract
Background: Major histocompatibility complex class I (MHCI) proteins present antigenic peptides for immune surveillance and play critical roles in nervous system development and plasticity. Most MHCI are transmembrane proteins. The extracellular domain of MHCI interacts with immunoreceptors, peptides, and co-receptors to mediate immune signaling. While the cytoplasmic domain also plays important roles in endocytic trafficking, cross-presentation of extracellularly derived antigens, and CTL priming, the molecular mediators of cytoplasmic signaling by MHCI remain largely unknown.
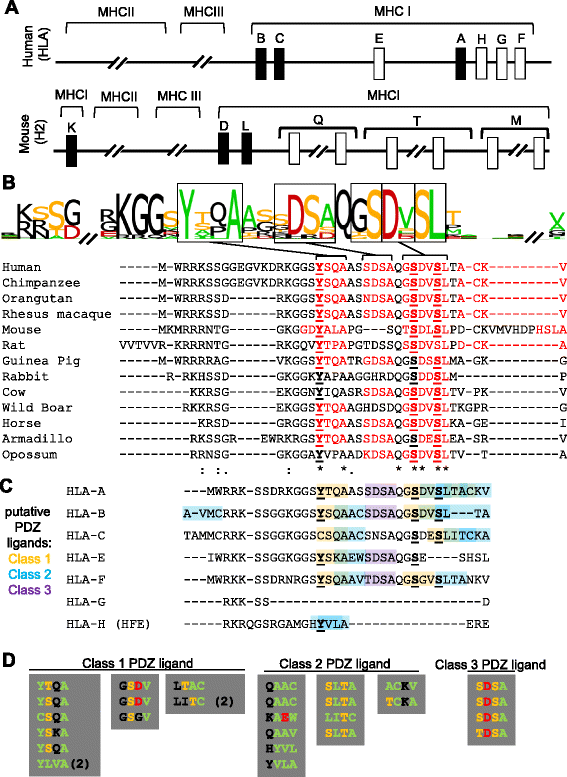
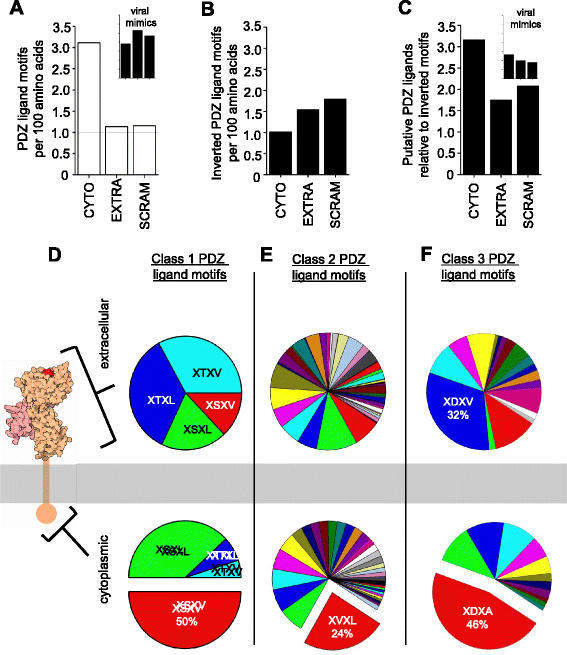
Results: Here we show that the cytoplasmic domain of MHCI contains putative protein-protein interaction domains known as PDZ (PSD95/disc large/zonula occludens-1) ligands. PDZ ligands are motifs that bind to PDZ domains to organize and mediate signaling at cell-cell contacts. PDZ ligands are short, degenerate motifs, and are therefore difficult to identify via sequence homology alone, but several lines of evidence suggest that putative PDZ ligand motifs in MHCI are under positive selective pressure. Putative PDZ ligands are found in all of the 99 MHCI proteins examined from diverse species, and are enriched in the cytoplasmic domain, where PDZ interactions occur. Both the position of the PDZ ligand and the class of ligand motif are conserved across species, as well as among genes within a species. Non-synonymous substitutions, when they occur, frequently preserve the motif. Of the many specific possible PDZ ligand motifs, a handful are strikingly and selectively overrepresented in MHCI's cytoplasmic domain, but not elsewhere in the same proteins. Putative PDZ ligands in MHCI encompass conserved serine and tyrosine residues that are targets of phosphorylation, a post-translational modification that can regulate PDZ interactions. Finally, proof-of-principle in vitro interaction assays demonstrate that the cytoplasmic domains of particular MHCI proteins can bind directly and specifically to PDZ1 and PDZ4&5 of MAGI-1, and identify a conserved PDZ ligand motif in the classical MHCI H2-K that is required for this interaction.
Conclusions: These results identify cryptic protein interaction motifs in the cytoplasmic domain of MHCI. In so doing, they suggest that the cytoplasmic domain of MHCI could participate in previously unsuspected PDZ mediated protein-protein interactions at neuronal as well as immunological synapses.
Keywords: Brain; Cytoplasmic; Immune; MHC class I; MHCI; PDZ; PDZ ligand; Scaffolding; Synapse.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

