Tert-butylhydroquinone lowers blood pressure in AngII-induced hypertension in mice via proteasome-PTEN-Akt-eNOS pathway
- PMID: 27435826
- PMCID: PMC4951646
- DOI: 10.1038/srep29589
Tert-butylhydroquinone lowers blood pressure in AngII-induced hypertension in mice via proteasome-PTEN-Akt-eNOS pathway
Retraction in
-
Retraction Note: Tert-butylhydroquinone lowers blood pressure in AngII-induced hypertension in mice via proteasome-PTEN-Akt-eNOS pathway.Sci Rep. 2019 Nov 19;9(1):17122. doi: 10.1038/s41598-019-53022-7. Sci Rep. 2019. PMID: 31745106 Free PMC article.
Abstract
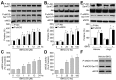
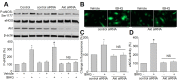
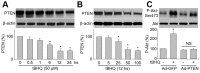
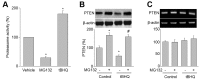
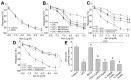
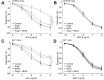
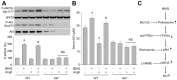
Tert-butylhydroquinone (tBHQ), as an antioxidant, has been widely used for many years to prevent oxidization of food products. The aim of this study was to investigate whether tBHQ activates endothelial nitric oxide synthase (eNOS) to prevent endothelial dysfunction and lower blood pressure. The role of Akt in tBHQ-induced eNOS phosphorylation was examined in human umbilical vein endothelial cells (HUVEC) or in mice. tBHQ treatment of HUVEC increased both Akt-Ser473 phosphorylation, accompanied with increased eNOS-Ser1177 phosphorylation and NO release. Mechanically, pharmacologic or genetic inhibition of Akt abolished tBHQ-enhanced NO release and eNOS phosphorylation in HUVEC. Gain-function of PTEN or inhibition of 26S proteasome abolished tBHQ-enhanced Akt phosphorylation in HUVEC. Ex vivo analysis indicated that tBHQ improved Ach-induced endothelium-dependent relaxation in LPC-treated mice aortic arteries, which were abolished by inhibition of Akt or eNOS. In animal study, administration of tBHQ significantly increased eNOS-Ser1177 phosphorylation and acetylcholine-induced vasorelaxation, and lowered AngII-induced hypertension in wildtype mice, but not in mice deficient of Akt or eNOS. In conclusion, tBHQ via proteasome-dependent degradation of PTEN increases Akt phosphorylation, resulting in upregulation of eNOS-derived NO production and consequent improvement of endothelial function in vivo. In this way, tBHQ lowers blood pressure in hypertensive mice.
Figures

References
-
- Dimmeler S. et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

