Glutathione peroxidase 3 localizes to the epithelial lining fluid and the extracellular matrix in interstitial lung disease
- PMID: 27435875
- PMCID: PMC4951690
- DOI: 10.1038/srep29952
Glutathione peroxidase 3 localizes to the epithelial lining fluid and the extracellular matrix in interstitial lung disease
Abstract
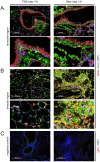
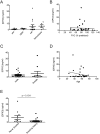
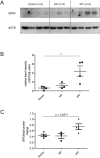
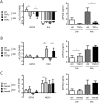
Aberrant antioxidant activity and excessive deposition of extracellular matrix (ECM) are hallmarks of interstitial lung diseases (ILD). It is known that oxidative stress alters the ECM, but extracellular antioxidant defence mechanisms in ILD are incompletely understood. Here, we extracted abundance and detergent solubility of extracellular antioxidant enzymes from a proteomic dataset of bleomycin-induced lung fibrosis in mice and assessed regulation and distribution of glutathione peroxidase 3 (GPX3) in murine and human lung fibrosis. Superoxide dismutase 3 (Sod3), Gpx3, and Gpx activity were increased in mouse BALF during bleomycin-induced lung fibrosis. In lung tissue homogenates, Gpx3, but not Sod3, was upregulated and detergent solubility profiling indicated that Gpx3 associated with ECM proteins. Immunofluorescence analysis showed that Gpx3 was expressed by bronchial epithelial cells and interstitial fibroblasts and localized to the basement membrane and interstitial ECM in lung tissue. As to human ILD samples, BALF of some patients contained high levels of GPX3, and GPX3 was upregulated in lung homogenates from IPF patients. GPX3 expression in primary human bronchial epithelial cells and lung fibroblasts was downregulated by TNF-α, but more variably regulated by TGF-β1 and menadione. In conclusion, the antioxidant enzyme GPX3 localizes to lung ECM and is variably upregulated in ILD.
Figures



Similar articles
-
Bach1 siRNA attenuates bleomycin-induced pulmonary fibrosis by modulating oxidative stress in mice.Int J Mol Med. 2017 Jan;39(1):91-100. doi: 10.3892/ijmm.2016.2823. Epub 2016 Dec 8. Int J Mol Med. 2017. PMID: 27959382 Free PMC article.
-
Superoxide Dismutase 3 R213G Single-Nucleotide Polymorphism Blocks Murine Bleomycin-Induced Fibrosis and Promotes Resolution of Inflammation.Am J Respir Cell Mol Biol. 2017 Mar;56(3):362-371. doi: 10.1165/rcmb.2016-0153OC. Am J Respir Cell Mol Biol. 2017. PMID: 27805412 Free PMC article.
-
The role of high mobility group box1 in pulmonary fibrosis.Am J Respir Cell Mol Biol. 2008 Oct;39(4):440-7. doi: 10.1165/rcmb.2007-0330OC. Epub 2008 Apr 25. Am J Respir Cell Mol Biol. 2008. PMID: 18441281
-
Extracellular superoxide dismutase in pulmonary fibrosis.Antioxid Redox Signal. 2008 Feb;10(2):343-54. doi: 10.1089/ars.2007.1908. Antioxid Redox Signal. 2008. PMID: 17999630 Free PMC article. Review.
-
Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer.Cancers (Basel). 2020 Aug 6;12(8):2197. doi: 10.3390/cancers12082197. Cancers (Basel). 2020. PMID: 32781581 Free PMC article. Review.
Cited by
-
New Strategies and Challenges in Lung Proteomics and Metabolomics. An Official American Thoracic Society Workshop Report.Ann Am Thorac Soc. 2017 Dec;14(12):1721-1743. doi: 10.1513/AnnalsATS.201710-770WS. Ann Am Thorac Soc. 2017. PMID: 29192815 Free PMC article.
-
Role of Mesenchymal Stem Cells and Extracellular Vesicles in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2022 Sep 23;23(19):11212. doi: 10.3390/ijms231911212. Int J Mol Sci. 2022. PMID: 36232511 Free PMC article. Review.
-
Severity of neonatal influenza infection is driven by type I interferon and oxidative stress.Mucosal Immunol. 2022 Jun;15(6):1309-1320. doi: 10.1038/s41385-022-00576-x. Epub 2022 Nov 9. Mucosal Immunol. 2022. PMID: 36352099 Free PMC article.
-
The Effects of Ionising and Non-Ionising Electromagnetic Radiation on Extracellular Matrix Proteins.Cells. 2021 Nov 5;10(11):3041. doi: 10.3390/cells10113041. Cells. 2021. PMID: 34831262 Free PMC article. Review.
-
Current and prospective applications of exosomal microRNAs in pulmonary fibrosis (Review).Int J Mol Med. 2022 Mar;49(3):37. doi: 10.3892/ijmm.2022.5092. Epub 2022 Jan 28. Int J Mol Med. 2022. PMID: 35088880 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

