Antibiotics Suppress Activation of Intestinal Mucosal Mast Cells and Reduce Dietary Lipid Absorption in Sprague-Dawley Rats
- PMID: 27436071
- PMCID: PMC5391873
- DOI: 10.1053/j.gastro.2016.07.009
Antibiotics Suppress Activation of Intestinal Mucosal Mast Cells and Reduce Dietary Lipid Absorption in Sprague-Dawley Rats
Abstract
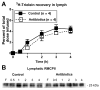
Background & aims: The gut microbiota affects intestinal permeability and mucosal mast cells (MMCs) responses. Activation of MMCs has been associated with absorption of dietary fat. We investigated whether the gut microbiota contributes to the fat-induced activation of MMCs in rats, and how antibiotics might affect this process.
Methods: Adult male Sprague-Dawley rats were given streptomycin and penicillin for 4 days (n = 6-8) to reduce the abundance of their gut flora, or normal drinking water (controls, n = 6-8). They underwent lymph fistula surgery and after an overnight recovery were given an intraduodenal bolus of intralipid. We collected intestinal tissues and lymph fluid and assessed activation of MMCs, intestinal permeability, and fat transport parameters.
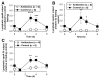
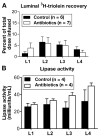
Results: Compared with controls, intestinal lymph from rats given antibiotics had reduced levels of mucosal mast cell protease II (produced by MMCs) and decreased activity of diamine oxidase (produced by enterocytes) (P < .05). Rats given antibiotics had reduced intestinal permeability in response to dietary lipid compared with controls (P < .01). Unexpectedly, antibiotics also reduced lymphatic transport of triacylglycerol and phospholipid (P < .01), concomitant with decreased levels of mucosal apolipoproteins B, A-I, and A-IV (P < .01). No differences were found in intestinal motility or luminal pancreatic lipase activity between rats given antibiotics and controls. These effects were not seen with an acute dose of antibiotics or 4 weeks after the antibiotic regimen ended.
Conclusions: The intestinal microbiota appears to activate MMCs after the ingestion of fat in rats; this contributes to fat-induced intestinal permeability. We found that the gut microbiome promotes absorption of lipid, probably by intestinal production of apolipoproteins and secretion of chylomicrons.
Keywords: APOA-I; APOA-IV; Digestion; Microbe.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors disclose no conflicts.
Figures

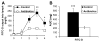
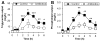

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

