Increased Metabotropic Glutamate Receptor 5 Signaling Underlies Obsessive-Compulsive Disorder-like Behavioral and Striatal Circuit Abnormalities in Mice
- PMID: 27436084
- PMCID: PMC5536332
- DOI: 10.1016/j.biopsych.2016.04.023
Increased Metabotropic Glutamate Receptor 5 Signaling Underlies Obsessive-Compulsive Disorder-like Behavioral and Striatal Circuit Abnormalities in Mice
Abstract
Background: Development of treatments for obsessive-compulsive disorder (OCD) is hampered by a lack of mechanistic understanding about this prevalent neuropsychiatric condition. Although circuit changes such as elevated frontostriatal activity are linked to OCD, the underlying molecular signaling that drives OCD-related behaviors remains largely unknown. Here, we examine the significance of type 5 metabotropic glutamate receptors (mGluR5s) for behavioral and circuit abnormalities relevant to OCD.
Methods: Sapap3 knockout (KO) mice treated acutely with an mGluR5 antagonist were evaluated for OCD-relevant phenotypes of self-grooming, anxiety-like behaviors, and increased striatal activity. The role of mGluR5 in the striatal circuit abnormalities of Sapap3 KO mice was further explored using two-photon calcium imaging to monitor striatal output from the direct and indirect pathways. A contribution of constitutive signaling to increased striatal mGluR5 activity in Sapap3 KO mice was investigated using pharmacologic and biochemical approaches. Finally, sufficiency of mGluR5 to drive OCD-like behavior in wild-type mice was tested by potentiating mGluR5 with a positive allosteric modulator.
Results: Excessive mGluR5 signaling underlies OCD-like behaviors and striatal circuit abnormalities in Sapap3 KO mice. Accordingly, enhancing mGluR5 activity acutely recapitulates these behavioral phenotypes in wild-type mice. In Sapap3 KO mice, elevated mGluR5 signaling is associated with constitutively active receptors and increased and imbalanced striatal output that is acutely corrected by antagonizing striatal mGluR5.
Conclusions: These findings demonstrate a causal role for increased mGluR5 signaling in driving striatal output abnormalities and behaviors with relevance to OCD and show the tractability of acute mGluR5 inhibition to remedy circuit and behavioral abnormalities.
Keywords: Circuit; Constitutive activity; Obsessive-compulsive disorder; Positive allosteric modulator; Striatum; mGluR5.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Figures


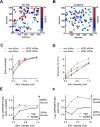
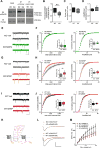

Comment in
-
Metabotropic Glutamate Receptor 5 as a Point of Convergence for Models of Obsessive-Compulsive Disorder and Autism Spectrum Disorder.Biol Psychiatry. 2016 Oct 1;80(7):504-6. doi: 10.1016/j.biopsych.2016.08.005. Biol Psychiatry. 2016. PMID: 27601339 No abstract available.
Similar articles
-
Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder.Biol Psychiatry. 2014 Apr 15;75(8):623-30. doi: 10.1016/j.biopsych.2013.01.008. Epub 2013 Feb 13. Biol Psychiatry. 2014. PMID: 23414593 Free PMC article.
-
Progression of obsessive compulsive disorder-like grooming in Sapap3 knockout mice: A longitudinal [11C]ABP688 PET study.Neuropharmacology. 2020 Oct 15;177:108160. doi: 10.1016/j.neuropharm.2020.108160. Epub 2020 May 23. Neuropharmacology. 2020. PMID: 32454126
-
Premorbid characteristics of the SAPAP3 mouse model of obsessive-compulsive disorder: behavior, neuroplasticity, and psilocybin treatment.Int J Neuropsychopharmacol. 2025 May 9;28(5):pyaf022. doi: 10.1093/ijnp/pyaf022. Int J Neuropsychopharmacol. 2025. PMID: 40156912 Free PMC article.
-
Molecular and cellular basis of obsessive-compulsive disorder-like behaviors: emerging view from mouse models.Curr Opin Neurol. 2011 Apr;24(2):114-8. doi: 10.1097/WCO.0b013e32834451fb. Curr Opin Neurol. 2011. PMID: 21386675 Review.
-
Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology.Psychiatr Clin North Am. 2006 Jun;29(2):371-90. doi: 10.1016/j.psc.2006.02.007. Psychiatr Clin North Am. 2006. PMID: 16650714 Review.
Cited by
-
Pathway-specific alterations in striatal excitability and cholinergic modulation in a SAPAP3 mouse model of compulsive motor behavior.Cell Rep. 2023 Nov 28;42(11):113384. doi: 10.1016/j.celrep.2023.113384. Epub 2023 Nov 6. Cell Rep. 2023. PMID: 37934666 Free PMC article.
-
Neuronal Glutamate Transporters Control Dopaminergic Signaling and Compulsive Behaviors.J Neurosci. 2018 Jan 24;38(4):937-961. doi: 10.1523/JNEUROSCI.1906-17.2017. Epub 2017 Dec 11. J Neurosci. 2018. PMID: 29229708 Free PMC article.
-
Activated CaMKIIα Binds to the mGlu5 Metabotropic Glutamate Receptor and Modulates Calcium Mobilization.Mol Pharmacol. 2018 Dec;94(6):1352-1362. doi: 10.1124/mol.118.113142. Epub 2018 Oct 3. Mol Pharmacol. 2018. PMID: 30282777 Free PMC article.
-
Glutamate spillover in C. elegans triggers repetitive behavior through presynaptic activation of MGL-2/mGluR5.Nat Commun. 2019 Apr 23;10(1):1882. doi: 10.1038/s41467-019-09581-4. Nat Commun. 2019. PMID: 31015396 Free PMC article.
-
Cell-type-specific disruption of cortico-striatal circuitry drives repetitive patterns of behavior in fragile X syndrome model mice.Cell Rep. 2023 Aug 29;42(8):112901. doi: 10.1016/j.celrep.2023.112901. Epub 2023 Jul 27. Cell Rep. 2023. PMID: 37505982 Free PMC article.
References
-
- McGuire JF, Lewin AB, Horng B, Murphy TK, Storch EA. The nature, assessment, and treatment of obsessive-compulsive disorder. Postgrad Med. 2012;124:152–165. - PubMed
-
- Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8:445–460. - PubMed
-
- Guehl D, Benazzouz A, Aouizerate B, Cuny E, Rotge JY, Rougier A, et al. Neuronal correlates of obsessions in the caudate nucleus. Biol Psychiatry. 2008;63:557–562. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

