Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use
- PMID: 27436286
- PMCID: PMC5009743
- DOI: 10.1093/nar/gkw530
Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use
Abstract
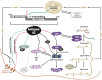
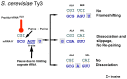
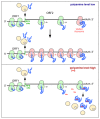
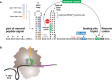
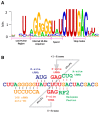
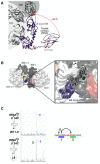
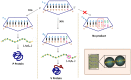
Genetic decoding is not 'frozen' as was earlier thought, but dynamic. One facet of this is frameshifting that often results in synthesis of a C-terminal region encoded by a new frame. Ribosomal frameshifting is utilized for the synthesis of additional products, for regulatory purposes and for translational 'correction' of problem or 'savior' indels. Utilization for synthesis of additional products occurs prominently in the decoding of mobile chromosomal element and viral genomes. One class of regulatory frameshifting of stable chromosomal genes governs cellular polyamine levels from yeasts to humans. In many cases of productively utilized frameshifting, the proportion of ribosomes that frameshift at a shift-prone site is enhanced by specific nascent peptide or mRNA context features. Such mRNA signals, which can be 5' or 3' of the shift site or both, can act by pairing with ribosomal RNA or as stem loops or pseudoknots even with one component being 4 kb 3' from the shift site. Transcriptional realignment at slippage-prone sequences also generates productively utilized products encoded trans-frame with respect to the genomic sequence. This too can be enhanced by nucleic acid structure. Together with dynamic codon redefinition, frameshifting is one of the forms of recoding that enriches gene expression.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Yanagitani K., Imagawa Y., Iwawaki T., Hosoda A., Saito M., Kimata Y., Kohno K. Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol. Cell. 2009;34:191–200. - PubMed
-
- Keiler K.C. Mechanisms of ribosome rescue in bacteria. Nat. Rev. Microbiol. 2015;13:285–297. - PubMed
-
- Himeno H., Nameki N., Kurita D., Muto A., Abo T. Ribosome rescue systems in bacteria. Biochimie. 2015;114:102–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

