Glycolytic regulation of cell rearrangement in angiogenesis
- PMID: 27436424
- PMCID: PMC4961802
- DOI: 10.1038/ncomms12240
Glycolytic regulation of cell rearrangement in angiogenesis
Abstract
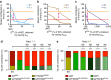
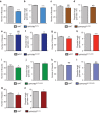
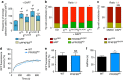
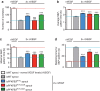
During vessel sprouting, endothelial cells (ECs) dynamically rearrange positions in the sprout to compete for the tip position. We recently identified a key role for the glycolytic activator PFKFB3 in vessel sprouting by regulating cytoskeleton remodelling, migration and tip cell competitiveness. It is, however, unknown how glycolysis regulates EC rearrangement during vessel sprouting. Here we report that computational simulations, validated by experimentation, predict that glycolytic production of ATP drives EC rearrangement by promoting filopodia formation and reducing intercellular adhesion. Notably, the simulations correctly predicted that blocking PFKFB3 normalizes the disturbed EC rearrangement in high VEGF conditions, as occurs during pathological angiogenesis. This interdisciplinary study integrates EC metabolism in vessel sprouting, yielding mechanistic insight in the control of vessel sprouting by glycolysis, and suggesting anti-glycolytic therapy for vessel normalization in cancer and non-malignant diseases.
Conflict of interest statement
P.C. declares being named as inventor on patent applications claiming subject matter related to the results described in this paper. The remaining authors declare no competing financial interests.
Figures



References
-
- Phng L. K. & Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196–208 (2009). - PubMed
-
- Potente M., Gerhardt H. & Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011). - PubMed
-
- Arima S. et al.. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 138, 4763–4776 (2011). - PubMed
-
- Jakobsson L. et al.. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943–953 (2010). - PubMed
-
- De Bock K. et al.. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

