Poor Mobilization in T-Cell-Deficient Nude Mice Is Explained by Defective Activation of Granulocytes and Monocytes
- PMID: 27436627
- PMCID: PMC5516889
- DOI: 10.3727/096368916X692221
Poor Mobilization in T-Cell-Deficient Nude Mice Is Explained by Defective Activation of Granulocytes and Monocytes
Abstract
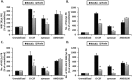
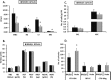
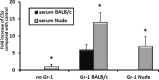
It has been reported that both SCID mice and SCID patients poorly mobilize hematopoietic stem/progenitor cells (HSPCs) in response to granulocyte colony-stimulating factor (G-CSF). This defect has been proposed to result from a lack of naturally occurring IgM immunoglobulins to trigger activation of the complement cascade (ComC) and release of C5 cleavage fragments crucial in the mobilization process. However, SCID individuals also have T-cell deficiency, and T cells have been shown to modulate trafficking of HSPCs. To learn more about the role of T lymphocytes, we performed mobilization studies in T-lymphocyte-deficient nude mice and found that these mice respond poorly to G-CSF and zymosan but are normal mobilizers in response to AMD3100. Since nude mice have normal levels of IgM immunoglobulins in peripheral blood and may activate the ComC, we focused on the potential involvement of Gr1+ granulocytes and monocytes, which show defective maturation in these animals. Using a nude mouse mobilization model, we found further support for the proposition that proper function of Gr1+ cells is crucial for optimal mobilization of HSPCs.
Figures


Similar articles
-
Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes.Leukemia. 2010 Mar;24(3):573-82. doi: 10.1038/leu.2009.271. Epub 2009 Dec 24. Leukemia. 2010. PMID: 20033053 Free PMC article.
-
Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes.Leukemia. 2009 Nov;23(11):2052-62. doi: 10.1038/leu.2009.158. Epub 2009 Aug 6. Leukemia. 2009. PMID: 19657368 Free PMC article.
-
A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins.Stem Cells. 2007 Dec;25(12):3093-100. doi: 10.1634/stemcells.2007-0525. Epub 2007 Aug 23. Stem Cells. 2007. PMID: 17717064
-
A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC).Adv Exp Med Biol. 2008;632:47-60. Adv Exp Med Biol. 2008. PMID: 19025113 Review.
-
In and out of the niche: perspectives in mobilization of hematopoietic stem cells.Exp Hematol. 2011 Jul;39(7):723-9. doi: 10.1016/j.exphem.2011.05.004. Epub 2011 May 13. Exp Hematol. 2011. PMID: 21624427 Review.
Cited by
-
Anticoagulants Interfere With the Angiogenic and Regenerative Responses Mediated by Platelets.Front Bioeng Biotechnol. 2020 Mar 20;8:223. doi: 10.3389/fbioe.2020.00223. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32266247 Free PMC article.
-
Inflammasomes and the Maintenance of Hematopoietic Homeostasis: New Perspectives and Opportunities.Molecules. 2021 Jan 9;26(2):309. doi: 10.3390/molecules26020309. Molecules. 2021. PMID: 33435298 Free PMC article. Review.
-
G-CSF-Induced Emergency Granulopoiesis Modulates Neutrophil Effector Function in Mice.Stem Cell Rev Rep. 2025 May;21(4):1113-1126. doi: 10.1007/s12015-025-10885-w. Epub 2025 Apr 29. Stem Cell Rev Rep. 2025. PMID: 40299198 Free PMC article.
-
Innate immunity orchestrates the mobilization and homing of hematopoietic stem/progenitor cells by engaging purinergic signaling-an update.Purinergic Signal. 2020 Jun;16(2):153-166. doi: 10.1007/s11302-020-09698-y. Epub 2020 May 15. Purinergic Signal. 2020. PMID: 32415576 Free PMC article. Review.
-
Phase partitioning of the neutrophil oxidative burst is coordinated by accessory pathways of glucose metabolism and mitochondrial activity.J Biol Chem. 2025 Jan;301(1):108091. doi: 10.1016/j.jbc.2024.108091. Epub 2024 Dec 13. J Biol Chem. 2025. PMID: 39675714 Free PMC article.
References
-
- Lévesque J.P., Helwani F.M., Winkler I.G.. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 2010; 24(12): 1979–92. - PubMed
-
- Lapidot T., Kollet O.. The brain-bone-blood triad: Traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program 2010; 2010: 1–6. - PubMed
-
- Ratajczak M.Z., Kim C.H., Wojakowski W., Janowska-Wieczorek A., Kucia M., Ratajczak J.. Innate immunity as orchestrator of stem cell mobilization. Leukemia 2010; 24(10): 1667–75. - PubMed
-
- Doan P.L., Chute J.P.. The vascular niche: Home for normal and malignant hematopoietic stem cells. Leukemia 2012; 26(1): 54–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

