Auto-fusion and the shaping of neurons and tubes
- PMID: 27436685
- PMCID: PMC5161574
- DOI: 10.1016/j.semcdb.2016.07.018
Auto-fusion and the shaping of neurons and tubes
Abstract
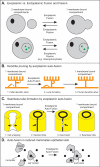
Cells adopt specific shapes that are necessary for specific functions. For example, some neurons extend elaborate arborized dendrites that can contact multiple targets. Epithelial and endothelial cells can form tiny seamless unicellular tubes with an intracellular lumen. Recent advances showed that cells can auto-fuse to acquire those specific shapes. During auto-fusion, a cell merges two parts of its own plasma membrane. In contrast to cell-cell fusion or macropinocytic fission, which result in the merging or formation of two separate membrane bound compartments, auto-fusion preserves one compartment, but changes its shape. The discovery of auto-fusion in C. elegans was enabled by identification of specific protein fusogens, EFF-1 and AFF-1, that mediate cell-cell fusion. Phenotypic characterization of eff-1 and aff-1 mutants revealed that fusogen-mediated fusion of two parts of the same cell can be used to sculpt dendritic arbors, reconnect two parts of an axon after injury, or form a hollow unicellular tube. Similar auto-fusion events recently were detected in vertebrate cells, suggesting that auto-fusion could be a widely used mechanism for shaping neurons and tubes.
Keywords: Auto-fusion; Dendrite morphogenesis; Fusogen; Neurons regeneration; Seamless tube.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Extrinsic Repair of Injured Dendrites as a Paradigm for Regeneration by Fusion in Caenorhabditis elegans.Genetics. 2017 May;206(1):215-230. doi: 10.1534/genetics.116.196386. Epub 2017 Mar 10. Genetics. 2017. PMID: 28283540 Free PMC article.
-
The AFF-1 exoplasmic fusogen is required for endocytic scission and seamless tube elongation.Nat Commun. 2018 May 1;9(1):1741. doi: 10.1038/s41467-018-04091-1. Nat Commun. 2018. PMID: 29717108 Free PMC article.
-
AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans.Dev Cell. 2007 May;12(5):683-98. doi: 10.1016/j.devcel.2007.03.003. Dev Cell. 2007. PMID: 17488621 Free PMC article.
-
Time to make the doughnuts: Building and shaping seamless tubes.Semin Cell Dev Biol. 2017 Jul;67:123-131. doi: 10.1016/j.semcdb.2016.05.006. Epub 2016 May 10. Semin Cell Dev Biol. 2017. PMID: 27178486 Free PMC article. Review.
-
Endocytosis regulates membrane localization and function of the fusogen EFF-1.Small GTPases. 2017 Jul 3;8(3):177-180. doi: 10.1080/21541248.2016.1211399. Epub 2016 Jul 28. Small GTPases. 2017. PMID: 27470417 Free PMC article. Review.
Cited by
-
Transcytosis in the development and morphogenesis of epithelial tissues.EMBO J. 2021 May 3;40(9):e106163. doi: 10.15252/embj.2020106163. Epub 2021 Apr 1. EMBO J. 2021. PMID: 33792936 Free PMC article. Review.
-
Phylogenetics of swimming behaviour in Medusozoa: the role of giant axons and their possible evolutionary origin.J Exp Biol. 2022 Mar 8;225(Suppl_1):jeb243382. doi: 10.1242/jeb.243382. Epub 2022 Mar 8. J Exp Biol. 2022. PMID: 35258622 Free PMC article.
-
How cells fuse.J Cell Biol. 2019 May 6;218(5):1436-1451. doi: 10.1083/jcb.201901017. Epub 2019 Apr 1. J Cell Biol. 2019. PMID: 30936162 Free PMC article. Review.
-
Engineering Cellular Self-Adhesions Inside 3D Printed Micro-Arches to Enhance Cell:Biomaterial Attachment.Adv Mater. 2025 Aug;37(32):e2502425. doi: 10.1002/adma.202502425. Epub 2025 May 24. Adv Mater. 2025. PMID: 40411865 Free PMC article.
-
Extrinsic Repair of Injured Dendrites as a Paradigm for Regeneration by Fusion in Caenorhabditis elegans.Genetics. 2017 May;206(1):215-230. doi: 10.1534/genetics.116.196386. Epub 2017 Mar 10. Genetics. 2017. PMID: 28283540 Free PMC article.
References
-
- Lefebvre JL, Sanes JR, Kay JN. Development of dendritic form and function. Annu Rev Cell Dev Biol. 2015;31:741–77. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

