The Arabidopsis SR45 Splicing Factor, a Negative Regulator of Sugar Signaling, Modulates SNF1-Related Protein Kinase 1 Stability
- PMID: 27436712
- PMCID: PMC5006706
- DOI: 10.1105/tpc.16.00301
The Arabidopsis SR45 Splicing Factor, a Negative Regulator of Sugar Signaling, Modulates SNF1-Related Protein Kinase 1 Stability
Abstract
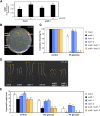
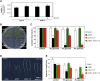
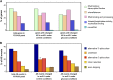
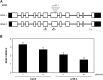
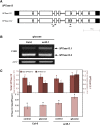
The ability to sense and respond to sugar signals allows plants to cope with environmental and metabolic changes by adjusting growth and development accordingly. We previously reported that the SR45 splicing factor negatively regulates glucose signaling during early seedling development in Arabidopsis thaliana Here, we show that under glucose-fed conditions, the Arabidopsis sr45-1 loss-of-function mutant contains higher amounts of the energy-sensing SNF1-Related Protein Kinase 1 (SnRK1) despite unaffected SnRK1 transcript levels. In agreement, marker genes for SnRK1 activity are upregulated in sr45-1 plants, and the glucose hypersensitivity of sr45-1 is attenuated by disruption of the SnRK1 gene. Using a high-resolution RT-PCR panel, we found that the sr45-1 mutation broadly targets alternative splicing in vivo, including that of the SR45 pre-mRNA itself. Importantly, the enhanced SnRK1 levels in sr45-1 are suppressed by a proteasome inhibitor, indicating that SR45 promotes targeting of the SnRK1 protein for proteasomal destruction. Finally, we demonstrate that SR45 regulates alternative splicing of the Arabidopsis 5PTase13 gene, which encodes an inositol polyphosphate 5-phosphatase previously shown to interact with and regulate the stability of SnRK1 in vitro, thus providing a mechanistic link between SR45 function and the modulation of degradation of the SnRK1 energy sensor in response to sugars.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures


References
-
- Aki T., Konishi M., Kikuchi T., Fujimori T., Yoneyama T., Yanagisawa S. (2007). Distinct modulations of the hexokinase1-mediated glucose response and hexokinase1-independent processes by HYS1/CPR5 in Arabidopsis. J. Exp. Bot. 58: 3239–3248. - PubMed
-
- Ali G.S., Reddy A.S. (2006). ATP, phosphorylation and transcription regulate the mobility of plant splicing factors. J. Cell Sci. 119: 3527–3538. - PubMed
-
- Ali G.S., Golovkin M., Reddy A.S. (2003). Nuclear localization and in vivo dynamics of a plant-specific serine/arginine-rich protein. Plant J. 36: 883–893. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

