Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants
- PMID: 27436713
- PMCID: PMC5006701
- DOI: 10.1105/tpc.16.00143
Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants
Abstract
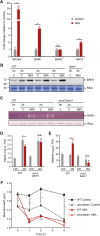
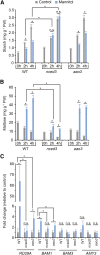
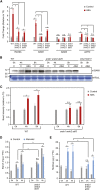
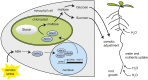
Starch serves functions that range over a timescale of minutes to years, according to the cell type from which it is derived. In guard cells, starch is rapidly mobilized by the synergistic action of β-AMYLASE1 (BAM1) and α-AMYLASE3 (AMY3) to promote stomatal opening. In the leaves, starch typically accumulates gradually during the day and is degraded at night by BAM3 to support heterotrophic metabolism. During osmotic stress, starch is degraded in the light by stress-activated BAM1 to release sugar and sugar-derived osmolytes. Here, we report that AMY3 is also involved in stress-induced starch degradation. Recently isolated Arabidopsis thaliana amy3 bam1 double mutants are hypersensitive to osmotic stress, showing impaired root growth. amy3 bam1 plants close their stomata under osmotic stress at similar rates as the wild type but fail to mobilize starch in the leaves. (14)C labeling showed that amy3 bam1 plants have reduced carbon export to the root, affecting osmolyte accumulation and root growth during stress. Using genetic approaches, we further demonstrate that abscisic acid controls the activity of BAM1 and AMY3 in leaves under osmotic stress through the AREB/ABF-SnRK2 kinase-signaling pathway. We propose that differential regulation and isoform subfunctionalization define starch-adaptive plasticity, ensuring an optimal carbon supply for continued growth under an ever-changing environment.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures
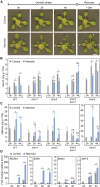
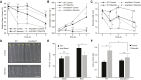
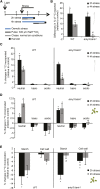
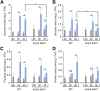

Comment in
-
Counting Carbs: Tracking Fluctuations in Starch-Derived Metabolite Levels Uncovers Their Crucial Roles in Osmotic Stress Tolerance.Plant Cell. 2016 Aug;28(8):1757-8. doi: 10.1105/tpc.16.00585. Epub 2016 Jul 27. Plant Cell. 2016. PMID: 27465025 Free PMC article. No abstract available.
Similar articles
-
β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress.J Exp Bot. 2016 Mar;67(6):1819-26. doi: 10.1093/jxb/erv572. Epub 2016 Jan 20. J Exp Bot. 2016. PMID: 26792489
-
Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress.J Exp Bot. 2011 Jan;62(2):545-55. doi: 10.1093/jxb/erq288. Epub 2010 Sep 27. J Exp Bot. 2011. PMID: 20876336 Free PMC article.
-
β-Amylase1 and β-amylase3 are plastidic starch hydrolases in Arabidopsis That Seem to Be Adapted for Different Thermal, pH, and stress conditions.Plant Physiol. 2014 Dec;166(4):1748-63. doi: 10.1104/pp.114.246421. Epub 2014 Oct 7. Plant Physiol. 2014. PMID: 25293962 Free PMC article.
-
Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants.Physiol Plant. 2013 Jan;147(1):15-27. doi: 10.1111/j.1399-3054.2012.01635.x. Epub 2012 May 16. Physiol Plant. 2013. PMID: 22519646 Review.
-
OPEN STOMATA1 opens the door to ABA signaling in Arabidopsis guard cells.Trends Plant Sci. 2003 Apr;8(4):151-3. doi: 10.1016/S1360-1385(03)00052-9. Trends Plant Sci. 2003. PMID: 12711225 Review.
Cited by
-
Responses of Manila Grass (Zoysia matrella) to chilling stress: From transcriptomics to physiology.PLoS One. 2020 Jul 20;15(7):e0235972. doi: 10.1371/journal.pone.0235972. eCollection 2020. PLoS One. 2020. PMID: 32687533 Free PMC article.
-
Transcriptomic and metabolomic profiling reveals the drought tolerance mechanism of Illicium difengpi (Schisandraceae).Front Plant Sci. 2024 Jan 8;14:1284135. doi: 10.3389/fpls.2023.1284135. eCollection 2023. Front Plant Sci. 2024. PMID: 38259923 Free PMC article.
-
Advances in Wheat Physiology in Response to Drought and the Role of Plant Growth Promoting Rhizobacteria to Trigger Drought Tolerance.Microorganisms. 2021 Mar 26;9(4):687. doi: 10.3390/microorganisms9040687. Microorganisms. 2021. PMID: 33810405 Free PMC article. Review.
-
Genome-Wide Investigation of BAM Gene Family in Annona atemoya: Evolution and Expression Network Profiles during Fruit Ripening.Int J Mol Sci. 2023 Jun 22;24(13):10516. doi: 10.3390/ijms241310516. Int J Mol Sci. 2023. PMID: 37445694 Free PMC article.
-
Unveiling a differential metabolite modulation of sorghum varieties under increasing tunicamycin-induced endoplasmic reticulum stress.Cell Stress Chaperones. 2023 Nov;28(6):889-907. doi: 10.1007/s12192-023-01382-5. Epub 2023 Sep 29. Cell Stress Chaperones. 2023. PMID: 37775652 Free PMC article.
References
-
- Baerenfaller K., Hirsch-Hoffmann M., Svozil J., Hull R., Russenberger D., Bischof S., Lu Q., Gruissem W., Baginsky S. (2011). pep2pro: a new tool for comprehensive proteome data analysis to reveal information about organ-specific proteomes in Arabidopsis thaliana. Integr. Biol. (Camb.) 3: 225–237. - PubMed
-
- Bartels D., Sunkar R. (2005). Drought and salt tolerance in plants. CRC. Crit. Rev. Plant Sci. 24: 23–58.
-
- Bates L.S., Waldren R.P., Teare I.D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207.
-
- Blatt M.R. (2016). Plant physiology: redefining the enigma of metabolism in stomatal movement. Curr. Biol. 26: R107–R109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

