Prostaglandin E2 receptor EP3 regulates both adipogenesis and lipolysis in mouse white adipose tissue
- PMID: 27436752
- PMCID: PMC5181317
- DOI: 10.1093/jmcb/mjw035
Prostaglandin E2 receptor EP3 regulates both adipogenesis and lipolysis in mouse white adipose tissue
Abstract
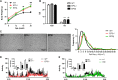
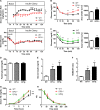
Among the four prostaglandin E2 receptors, EP3 receptor is the one most abundantly expressed in white adipose tissue (WAT). The mouse EP3 gene gives rise to three isoforms, namely EP3α, EP3β, and EP3γ, which differ only at their C-terminal tails. To date, functions of EP3 receptor and its isoforms in WAT remain incompletely characterized. In this study, we found that the expression of all EP3 isoforms were downregulated in WAT of both db/db and high-fat diet-induced obese mice. Genetic ablation of three EP3 receptor isoforms (EP3-/- mice) or EP3α and EP3γ isoforms with EP3β intact (EP3β mice) led to an obese phenotype with increased food intake, decreased motor activity, reduced insulin sensitivity, and elevated serum triglycerides. Since the differentiation of preadipocytes and mouse embryonic fibroblasts to adipocytes was markedly facilitated by either pharmacological blockade or genetic deletion/inhibition of EP3 receptor via the cAMP/PKA/PPARγ pathway, increased adipogenesis may contribute to obesity in EP3-/- and EP3β mice. Moreover, both EP3-/- and EP3β mice had increased lipolysis in WAT mainly due to the activated cAMP/PKA/hormone-sensitive lipase pathway. Taken together, our findings suggest that EP3 receptor and its α and γ isoforms are involved in both adipogenesis and lipolysis and influence food intake, serum lipid levels, and insulin sensitivity.
Keywords: EP3 receptor isoform; PKA; PPARγ; arachidonic acid; insulin resistance; obesity.
© The Author (2016). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. All rights reserved.
Figures




References
-
- Arner P., and Langin D. (2014). Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 25, 255–262. - PubMed
-
- Bastard J.P., Maachi M., Lagathu C., et al. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17, 4–12. - PubMed
-
- Borglum J.D., Pedersen S.B., Ailhaud G., et al. (1999). Differential expression of prostaglandin receptor mRNAs during adipose cell differentiation. Prostaglandins Other Lipid Mediat. 57, 305–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

