Impaired neuronal KCC2 function by biallelic SLC12A5 mutations in migrating focal seizures and severe developmental delay
- PMID: 27436767
- PMCID: PMC4951812
- DOI: 10.1038/srep30072
Impaired neuronal KCC2 function by biallelic SLC12A5 mutations in migrating focal seizures and severe developmental delay
Abstract
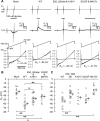
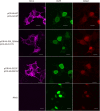
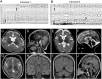
Epilepsy of infancy with migrating focal seizures (EIMFS) is one of the early-onset epileptic syndromes characterized by migrating polymorphous focal seizures. Whole exome sequencing (WES) in ten sporadic and one familial case of EIMFS revealed compound heterozygous SLC12A5 (encoding the neuronal K(+)-Cl(-) co-transporter KCC2) mutations in two families: c.279 + 1G > C causing skipping of exon 3 in the transcript (p.E50_Q93del) and c.572 C >T (p.A191V) in individuals 1 and 2, and c.967T > C (p.S323P) and c.1243 A > G (p.M415V) in individual 3. Another patient (individual 4) with migrating multifocal seizures and compound heterozygous mutations [c.953G > C (p.W318S) and c.2242_2244del (p.S748del)] was identified by searching WES data from 526 patients and SLC12A5-targeted resequencing data from 141 patients with infantile epilepsy. Gramicidin-perforated patch-clamp analysis demonstrated strongly suppressed Cl(-) extrusion function of E50_Q93del and M415V mutants, with mildly impaired function of A191V and S323P mutants. Cell surface expression levels of these KCC2 mutants were similar to wildtype KCC2. Heterologous expression of two KCC2 mutants, mimicking the patient status, produced a significantly greater intracellular Cl(-) level than with wildtype KCC2, but less than without KCC2. These data clearly demonstrated that partially disrupted neuronal Cl(-) extrusion, mediated by two types of differentially impaired KCC2 mutant in an individual, causes EIMFS.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

